Professional Documents
Culture Documents
DIY LED Photometer With Arduino For Physics or Che
DIY LED Photometer With Arduino For Physics or Che
Uploaded by
sako mooOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
DIY LED Photometer With Arduino For Physics or Che
DIY LED Photometer With Arduino For Physics or Che
Uploaded by
sako mooCopyright:
Available Formats
instructables
DIY LED-photometer With Arduino for Physics or Chemistry Lessons
by stoppi71
Hello! placed in the beam path and again measures the light
intensity or voltage U. The transmission factor in percent
Liquids or other objects appear colored because they is then simply calculated by T = U / U0 * 100. To get the
re ect or transmit certain colors and in turn swallow absorption-factor A you just have to calculate A = 100
(absorb) others. With a so-called photometer, those minus T.
colors (wavelengths) can be determined, which are
absorbed by liquids. The basic principle is simple: with a This measurement is repeated with di erently colored
LED of a certain color you rst shine through a cuvette LEDs and determines in each case T or A as a function of
lled with water or another solvent. A photodiode the wavelength (color). If you do this with enough LEDs,
measures the incoming light intensity and converts it you get an absorption curve.
into a proportional voltage U0. This value is noted.
Thereafter, a cuvette with the liquid to be examined is
DIY LED-photometer With Arduino for Physics or Chemistry Lessons: Page 1
Step 1: The Parts
For the photometer you need the following parts: * 6 resistors with 100, 1k, 10k, 100k, 1M and 10M ohms:
ebay resistors
* A black case with the dimensions 160 x 100 x 70 mm or * an I²C 16x2 display: ebay 16x2 display
similar: housing
* a 2x6 rotary switch: rotary switch
* An Arduino Nano: ebay arduino nano
* a 9V battery holder and a 9V battery: battery holder
* An operational ampli er LF356: ebay LF356
* a switch: switch
* 3 capacitors with a capacity of 10μF: ebay capacitors
* Glass cuvettes: ebay cuvettes
* 2 capacitors with C = 100nF and a capacitor with 1nF:
ebay capacitors * LEDs with di erent color: f.e. ebay LEDs
* One voltage inverter ICL7660: ebay ICL7660 * a simple 0-15V power supply to power the LEDs
* One photodiode BPW34: ebay BPW34 photodiode * wood for the cuvette-holder
DIY LED-photometer With Arduino for Physics or Chemistry Lessons: Page 2
DIY LED-photometer With Arduino for Physics or Chemistry Lessons: Page 3
DIY LED-photometer With Arduino for Physics or Chemistry Lessons: Page 4
DIY LED-photometer With Arduino for Physics or Chemistry Lessons: Page 5
DIY LED-photometer With Arduino for Physics or Chemistry Lessons: Page 6
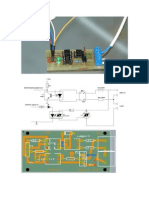
Step 2: The Circuit and the Arduino-code
DIY LED-photometer With Arduino for Physics or Chemistry Lessons: Page 7
The circuit for the photometer is very simple. It consists the resistor in the feedback of the OPA. To be more
of a photodiode, an operational ampli er, a voltage- exible I took 6 di erent resistors, which can be chosen
inverter and some other parts (resistors, switches, with the rotary switch. The lowest "magni cation" is 100,
capacitors). The principle of this type of circuit is to the highest 10 000 000. Everything is powered by a
convert the (low) current from the photodiode into a single 9V battery.
higher voltage, which can be read by the arduino nano.
The multiplication-factor is determined by the value of
Download
https://www.instructables.com/ORIG/FPG/T2KD/JVE7OL13/FPGT2KDJVE7OL13.ino
Step 3: First Experiment: the Absorption-curve of Chlorophyll
DIY LED-photometer With Arduino for Physics or Chemistry Lessons: Page 8
For the measuring procedure: A cuvette is lled with quite good. When selecting the LEDs, you should
water or another achieve as even coverage of the wavelength range from
transparent solvent. This is then placed in the 395nm to 850nm.
photometer. The cuvette is being covered with a light-
tight lid. Now set the power supply for the LED so that a For the rst experiment with the photometer I chose
current of about 10-20mA ows through the LED. After chlorophyll. But for this you’ll have to pluck grass from a
that, use the rotary switch to select the position at which meadow hoping that no one is watching you ...
the output voltage of the photodiode is around 3-4V.
The ne tuning of the output voltage can still be done This grass is then cut into small pieces and put together
with the adjustable power supply. This voltage U0 is with propanol or ethanol in a pot. Now you crush the
noted. Then take the cuvette containing the liquid to be leaves with a mortar or a fork. After a few minutes, the
examined and place it in the photometer. At this point chlorophyll has dissolved nicely in the propanol. This
the voltage of the power supply and the position of the solution is still too strong. It needs to be diluted with
rotary switch must remain unchanged! Then cover the su cient propanol. And to avoid any suspended the
cuvette again with the lid and measure the voltage U. solution has to be ltered. I took a common co ee- lter.
For the transmission T in percent the value is T = U / U0 *
100. To get the absorption coe cient A you just have to The result should look like as shown in the picture. A
calculate A = 100 - T. very translucent green-yellowish solution. Then you
repeat the measurement (U0, U) with each LED. As it can
I bought the di erent colored LEDs from Roithner be seen from the obtained absorption curve, theory and
Lasertechnik which is located in austria, my home measurement agree quite well. Chlorophyll a + b
country. For these, the respective wavelength is given in absorbs very strongly in the blue and red spectral range,
nanometers. To be really sure one can check the while green-yellow and infrared light can penetrate the
dominant wavelength with a spectroscope and the solution almost unhindered. In the infrared range, the
Theremino software (theremino spectrometer). In my absorption is even close to zero.
case, the data in nm agreed with the measurements
DIY LED-photometer With Arduino for Physics or Chemistry Lessons: Page 9
DIY LED-photometer With Arduino for Physics or Chemistry Lessons: Page 10
DIY LED-photometer With Arduino for Physics or Chemistry Lessons: Page 11
DIY LED-photometer With Arduino for Physics or Chemistry Lessons: Page 12
Step 4: Second Experiment: the Dependence of the Extinction on the Concentration of
Potassium Permanganate
DIY LED-photometer With Arduino for Physics or Chemistry Lessons: Page 13
As a further experiment, the determination of the As you can see from my extinction curve, it is not linear.
extinction depending on At higher concentrations, it attens, speci cally from
the concentration of the solute o ers. As a solute, I use concentrations greater than 0.25. This means that the
potassium permanganate. The light intensity after extinction is lower than would be expected according to
penetrating the solution follows the Lambert-Beer law: the Lambert-Beer law. However, considering only lower
It reads I = I0 * 10 ^ (- E). I0 is the intensity without solute, concentrations, for example between 0 and 0.25, results
I the intensity with solute and E the so-called extinction. in a very nice linear relationship between the
This extinction E depends (linearly) on the thickness x of concentration c and the extinction E. In this range, the
the cuvette and on the concentration c of the solute. unknown concentration c can be determined from the
Thus, E = k * c * x with k as the molar absorption measured extinction E. In my case, the concentration has
coe cient. To determine the extinction E you just need I only arbitrary units, since I have not determined the
and I0, because E = lg (I0 / I). When the intensity is initial amount of dissolved potassium permanganate (it
reduced to, for example, 10%, the extinction E = 1 (10 ^ - has been only milligrams, which couldn’t be measured
1). With a weakening to only 1%, E = 2 (10 ^ -2). with my kitchen-scale in my case, dissolved in 4 ml water
for the starting solution).
If one applies E as a function of the concentration c, we
would expect to obtain a rising straight line through the
zero point.
DIY LED-photometer With Arduino for Physics or Chemistry Lessons: Page 14
Step 5: Conclusions
DIY LED-photometer With Arduino for Physics or Chemistry Lessons: Page 15
This photometer is particularly suitable for physics and Eureka!
chemistry lessons.
The total cost is only around 60 Euro = 70 USD. The Last but not least I'd be very happy if you could vote for
di erent colored LEDs are the most expensive part. On me in the classroom-science-contest. Thank's for that...
ebay or aliexpress you will certainly nd cheaper LEDs
but usually you do not know which wavelengths the And if you are interested in further physics experiments,
LEDs have. Seen in this way, purchasing from a specialist here's my youtube-channel:
retailer is recommended.
https://www.youtube.com/user/stopperl16/videos?
In this lesson you learn something about the relation
between the colour of liquids and their absorption-
behavior, about the important Chlorophyll, the more physics projects: https://stoppi-homemade-
Lambert-Beer law, exponentials, transmission and physics.de/
absorption, calculation of percents and the wavelengths
of the visible colours. I think this is quite a lot...
So have fun also making this project in your lesson and
https://www.youtube.com/watch?v=SoVuUctSyBE
I saw the Youtube video. In the video you used 11 different colour LED's to make 11
measurements (1 per wavelength), but your excel plot shows 18 measurements at 18 different
wavelengths. How did you measure the other 7 wavelengths?
Hi Jamie!
First my compliment, you looked very accurate... I've ordered 12 LEDs from Roithner
Lasertechnik, the remaining 6 I had already at home. So no miracle or scam ;-)
Good luck with your photometer, cheers Christoph (stoppi)
Ah I see! I like this method, much less uncertainty than using a diffraction grating. An excellent
project, well done!
Hello. Very nice job. As you wrote RGB are just three wavelength LEDs, and colors we see are
just a perception. Which colors (or wavelength, it's better) do you advice to use?
Hi! You can see those LEDs I'm using for this photometer in the pictures. I've bought them from
Roithner Lasertechnik, located in vienna (austria). With those LEDs I cover the whole spectrum
from 400nm to 850nm well. I tried to have just 20-30nm gaps between the wavelengths. On ebay
you get 850nm IR, 395nm UV and some others. But f.e. I haven't found 5mm LEDs with lambda
between 660 and 760nm. For these LEDs a Laser-LED-company would be the right choice.
There are some companies like Roithner Lasertechnik in the USA for sure...
Thank you for your advice. Since I'm in Italy, Austria should be my best choice. Of course you got
my vote. Keep going on this way.
A GREAT JOB.. THANKS FOR SHARING.. GOT MY VOTE!..
thank's a lot ;-)
Very nice project indeed. It will be very helpful if you may be able to demonstrate it on Youtube!!
Hello! You can see the way how to use it on youtube, just look at the video at the end...
Nice project.
DIY LED-photometer With Arduino for Physics or Chemistry Lessons: Page 16
Rather than use a series of different LEDs would it not be possible to drive an RGB LED from
your Arduino and have the Arduino run the single diode through the entire color scale?
I think the quality of the wavelength for RGBw are not as precise.
Hi! When you use a RGB-Led you don't have different wavelengths even when the color
changes. For example when the red and green led are on, then you get a yellow but no yellow
wavelength. Therefore it isn't meaningful to use a RGB-Led. Better than so many different LEDs
would be a continuous spectrum, which you would get with a monochromator consisting of a
white light source, a f.e. Diffraction grating and slits. But on the other hand the light intensity of
each wavelength would be much lower then my different LEDs and the setup would be more
complicated
nice work - I like it!
Thank's...
DIY LED-photometer With Arduino for Physics or Chemistry Lessons: Page 17
You might also like
- Fun Circuit Experiments With Light and Soundsrev2Document43 pagesFun Circuit Experiments With Light and Soundsrev2vjeran bachNo ratings yet
- ECE 3074 AC Circuits Lab Lab 2: Winding Your Own Inductors & Making AC Measurements With An OscilloscopeDocument6 pagesECE 3074 AC Circuits Lab Lab 2: Winding Your Own Inductors & Making AC Measurements With An OscilloscopeZiad Mohmed FawzyNo ratings yet
- Diode (Lab Report)Document12 pagesDiode (Lab Report)Benjamin Cooper50% (6)
- Lab Report 227Document24 pagesLab Report 227lolaNo ratings yet
- SpectrophotometryDocument5 pagesSpectrophotometryKashifntcNo ratings yet
- DIY LED-photometer With Arduino For Physics or Chemistry LessonsDocument17 pagesDIY LED-photometer With Arduino For Physics or Chemistry Lessonskayito40No ratings yet
- The Photoelectric Effect Using LEDs As Light Sources PDFDocument4 pagesThe Photoelectric Effect Using LEDs As Light Sources PDFPriyal GuptaNo ratings yet
- Experiment No. 14 Planck's Constant (Photo Electric Effect)Document6 pagesExperiment No. 14 Planck's Constant (Photo Electric Effect)Somdyuti momoNo ratings yet
- Experiment No. 14 Planck's Constant Measuring With FiltersDocument6 pagesExperiment No. 14 Planck's Constant Measuring With FiltersSarveenaNo ratings yet
- Experiment 14 The Photoelectric Effect: ObjectiveDocument4 pagesExperiment 14 The Photoelectric Effect: ObjectiveHusnain RazaNo ratings yet
- Manual2 Planck's ConstantDocument6 pagesManual2 Planck's ConstantRagni RanjanNo ratings yet
- 212L 11 - Spectrum of HydrogenDocument3 pages212L 11 - Spectrum of Hydrogenqblaq18No ratings yet
- Plank's Constant LABDocument3 pagesPlank's Constant LABdheeraj ShahNo ratings yet
- Faculty of Engineering Electrical Department: Experiment 1 Pengukuran Paramiter Transistor HybridDocument18 pagesFaculty of Engineering Electrical Department: Experiment 1 Pengukuran Paramiter Transistor HybridSyieda ZamryNo ratings yet
- 14a Plancks Constant Photo Electric EffectDocument7 pages14a Plancks Constant Photo Electric EffectSAKSHI SINGHNo ratings yet
- Stefan Boltzmann DerivationDocument4 pagesStefan Boltzmann DerivationVlado VargasNo ratings yet
- AcknowledgementDocument18 pagesAcknowledgementRahul PantNo ratings yet
- PHOTOCONDUCTIVITYDocument39 pagesPHOTOCONDUCTIVITYSuhaila ctNo ratings yet
- Determination of Planck's Constant: Objectives of The ExperimentDocument5 pagesDetermination of Planck's Constant: Objectives of The ExperimentkishoreputhaNo ratings yet
- Activities 12 2022 UPDATEDDocument13 pagesActivities 12 2022 UPDATEDDZ GamesNo ratings yet
- Angel D. Acevedo Ross Intermediate Lab LL Lab Report 1: Specific Charge of Electron E/m Universidad de Puerto Rico: Rio PiedrasDocument9 pagesAngel D. Acevedo Ross Intermediate Lab LL Lab Report 1: Specific Charge of Electron E/m Universidad de Puerto Rico: Rio PiedrasangelNo ratings yet
- Physics 2 WL6Document7 pagesPhysics 2 WL6Braden WellsNo ratings yet
- Generation and Recombinations of Charge and LED SDocument23 pagesGeneration and Recombinations of Charge and LED SSaivivek PotnuruNo ratings yet
- The Worldwide KAPAGEN Successful ReplicationsDocument21 pagesThe Worldwide KAPAGEN Successful ReplicationsVlad AdrianNo ratings yet
- Lab Report 1Document10 pagesLab Report 1Sam ZahzouhiNo ratings yet
- Lab ManualDocument83 pagesLab ManualLucas EtienneNo ratings yet
- Experiment 21A Faraday'S LawDocument10 pagesExperiment 21A Faraday'S LawGreen ManNo ratings yet
- Introduction To Electrical Engineering I EE111 Introduction To Electrical Engineering I EE111Document1 pageIntroduction To Electrical Engineering I EE111 Introduction To Electrical Engineering I EE111Abdul Halil AbdullahNo ratings yet
- Simple Autorange Capacitor Tester / Capacitance Meter With Arduino and by HandDocument7 pagesSimple Autorange Capacitor Tester / Capacitance Meter With Arduino and by HandOussama LabsailiNo ratings yet
- Experiment 2 Aim: To Investigate The Kerr Effect Using Nitrobenzene. Apparatus:-Prism Table, Small Optical Bench, Pair of Polarizing FiltersDocument5 pagesExperiment 2 Aim: To Investigate The Kerr Effect Using Nitrobenzene. Apparatus:-Prism Table, Small Optical Bench, Pair of Polarizing FiltersSarveenaNo ratings yet
- Radiation Laws A2: Head of Experiment: Yvonne UnruhDocument12 pagesRadiation Laws A2: Head of Experiment: Yvonne UnruhShivani TiwariNo ratings yet
- Experiment#6 - Characteristic Curves of A Solar CellDocument20 pagesExperiment#6 - Characteristic Curves of A Solar Cellmuzahir.ali.baloch2021No ratings yet
- Connecting A 12V Relay To ArduinoDocument9 pagesConnecting A 12V Relay To Arduinojdabs666No ratings yet
- Opamp Rectifier & ClamperDocument26 pagesOpamp Rectifier & ClamperDebashish PalNo ratings yet
- Activity Notes BookDocument12 pagesActivity Notes Bookkaran singh class 11 aNo ratings yet
- Installation AllDocument45 pagesInstallation AllDaaniyyee AbdiisaaNo ratings yet
- Lesson 1452, Optoelectronics: Experiment 6, Photodiode and Phototransistor Current MeasurementsDocument30 pagesLesson 1452, Optoelectronics: Experiment 6, Photodiode and Phototransistor Current MeasurementsMagaNo ratings yet
- Experiment No. 4 Aim: ApparatusDocument4 pagesExperiment No. 4 Aim: ApparatusConqueror 101No ratings yet
- Arduino Controlled Light DimmerDocument21 pagesArduino Controlled Light Dimmerrodolfos_8No ratings yet
- Presentation EletricDocument19 pagesPresentation Eletricapi-295865486No ratings yet
- Exp. 2. Photo Electric EffectDocument6 pagesExp. 2. Photo Electric EffectMounika SaiNo ratings yet
- The Right Way To Test OptoisolatorDocument5 pagesThe Right Way To Test OptoisolatorMadhavesh Kulkarni100% (1)
- Lebanese International University School of EngineeringDocument12 pagesLebanese International University School of EngineeringOmar SroujiNo ratings yet
- Determining Plancks ConstantDocument4 pagesDetermining Plancks ConstantSimi ChNo ratings yet
- Planck's Constant LABDocument3 pagesPlanck's Constant LAByaduvanshilaxman321No ratings yet
- Viva Voice 2 Determining Planks ConstantDocument2 pagesViva Voice 2 Determining Planks ConstantMirza Shahzaib ShahidNo ratings yet
- A Simple DIY SpectrophotometerDocument30 pagesA Simple DIY SpectrophotometerMiguelDelBarrioIglesisas100% (1)
- A Binder Manual For PHY-307 July-DecDocument66 pagesA Binder Manual For PHY-307 July-DecAayush RandeepNo ratings yet
- V2CH12 Detecting LightDocument37 pagesV2CH12 Detecting LightKaro Pand FloresNo ratings yet
- About Optoisolator IC and The Right Way To Test It: ComputerDocument14 pagesAbout Optoisolator IC and The Right Way To Test It: ComputerJamal JardelNo ratings yet
- Arduino Controlled Light Dimmer: InstructablesDocument34 pagesArduino Controlled Light Dimmer: InstructablesAmarlo EuNo ratings yet
- Presentation EletricDocument19 pagesPresentation Eletricapi-300102785No ratings yet
- Experiment PHE2 - Characteristics of A Solar CellDocument12 pagesExperiment PHE2 - Characteristics of A Solar Cellmuzahir.ali.baloch2021No ratings yet
- Radiatergdfgadgergeg 43trf 34frefDocument10 pagesRadiatergdfgadgergeg 43trf 34frefjack jackNo ratings yet
- Experiment 1 Full Lab 1 3Document17 pagesExperiment 1 Full Lab 1 3api-306888788No ratings yet
- Measuring Planck's Constant Using Light Emitting Diodes (LED's)Document19 pagesMeasuring Planck's Constant Using Light Emitting Diodes (LED's)Jatin SinghNo ratings yet
- Color I MeterDocument3 pagesColor I MeterJoe Reilly100% (1)
- RWEP Final9 14 Assessment Questions v5Document7 pagesRWEP Final9 14 Assessment Questions v5K RamakrishnanNo ratings yet
- Medical Lab InstrumentDocument44 pagesMedical Lab InstrumentSujith MayakrishnanNo ratings yet
- Easy(er) Electrical Principles for General Class Ham License (2015-2019)From EverandEasy(er) Electrical Principles for General Class Ham License (2015-2019)Rating: 5 out of 5 stars5/5 (1)
- Cambium PTP 820 Tuning Guide PDFDocument11 pagesCambium PTP 820 Tuning Guide PDFEze Alexander IkNo ratings yet
- Heat Shrink Termination IXSU-F - OXSU-FDocument10 pagesHeat Shrink Termination IXSU-F - OXSU-Fdes1982No ratings yet
- Tesca Technologies Pvt. LTD.: Order Code - 52053 PC Based Analog & Digital Motor Control TrainerDocument4 pagesTesca Technologies Pvt. LTD.: Order Code - 52053 PC Based Analog & Digital Motor Control TrainerGoran MiljkovicNo ratings yet
- Manual de ServicioDocument133 pagesManual de ServicioMariano OhienartNo ratings yet
- Model 6300 and 6320D Spectrophotometer: Operating ManualDocument30 pagesModel 6300 and 6320D Spectrophotometer: Operating ManualdangthanhlinhNo ratings yet
- 7.voltage Drop and Power Loss CalculationDocument16 pages7.voltage Drop and Power Loss CalculationImjusttryingtohelpNo ratings yet
- Bell Canada ProgramDocument5 pagesBell Canada ProgramKrishnaKumar BrahmarouthuNo ratings yet
- AS1700-NPN and AS1700-PWRDocument21 pagesAS1700-NPN and AS1700-PWRWellison RodriguesNo ratings yet
- The Automatic Transmit Power ControlDocument2 pagesThe Automatic Transmit Power ControlhastimaheshrajuNo ratings yet
- API Recip Compressor Best Practices 0300 - 060814Document25 pagesAPI Recip Compressor Best Practices 0300 - 060814daniel adamNo ratings yet
- Eplan Software & Service GMBH & Co. KG: Ed Iec - Tpl001 Asus Date 2/4/2021Document13 pagesEplan Software & Service GMBH & Co. KG: Ed Iec - Tpl001 Asus Date 2/4/2021Tinto TenNo ratings yet
- Bus Duct Trunking SystemDocument15 pagesBus Duct Trunking SystemmahmoudNo ratings yet
- TI Designs TIDA-01243 USB Type-CTM Mini Dock: Design Overview Block DiagramDocument84 pagesTI Designs TIDA-01243 USB Type-CTM Mini Dock: Design Overview Block DiagramAlex LuzNo ratings yet
- Troubleshooting Storage Area NetworkDocument30 pagesTroubleshooting Storage Area NetworkAsif MahbubNo ratings yet
- An Vibration Ed2Rev0 English 0308Document4 pagesAn Vibration Ed2Rev0 English 0308indraandikapNo ratings yet
- Robo PDFDocument2 pagesRobo PDFRajamuthamilselvan MarimuthuNo ratings yet
- Megger Testing Method StatementDocument7 pagesMegger Testing Method StatementRay Agacia0% (1)
- NP RF511 PDFDocument48 pagesNP RF511 PDFAnderson FerraroNo ratings yet
- EU D2600 - D2650 Duet2 - 3 Combined Installation Manual v1 220110Document32 pagesEU D2600 - D2650 Duet2 - 3 Combined Installation Manual v1 220110Martin Alejandro Carretero VilarNo ratings yet
- APL5508/5508R/5509/5509R: Features General DescriptionDocument16 pagesAPL5508/5508R/5509/5509R: Features General DescriptionnhbrNo ratings yet
- MIP Lab Semester Project - Section EDocument5 pagesMIP Lab Semester Project - Section EAbdurrahman KhanNo ratings yet
- Delta Ia-Tc Dte10t Om en 20190122Document14 pagesDelta Ia-Tc Dte10t Om en 20190122FernandoNo ratings yet
- Sia-2018 NDT HandoutDocument17 pagesSia-2018 NDT HandoutSingh CVNo ratings yet
- NIKKON HID Lighting Catalogue PDFDocument95 pagesNIKKON HID Lighting Catalogue PDFIswadi Bin ZulkarnainNo ratings yet
- Cyber Space February 22, 2015Document6 pagesCyber Space February 22, 2015BabuKumarNo ratings yet
- Wandboard UserGuideDocument26 pagesWandboard UserGuideabeeshNo ratings yet
- MS - Bomba de Infusión JMS OT-701Document124 pagesMS - Bomba de Infusión JMS OT-701HisoriniNo ratings yet
- The Discographer - October 2013 - 2Document140 pagesThe Discographer - October 2013 - 278rpmcommunity100% (1)