Professional Documents
Culture Documents
Assignment 1
Assignment 1
Uploaded by
loli Xxxx0 ratings0% found this document useful (0 votes)
10 views1 pageThe document describes a gas enclosed in a cylinder where the initial volume is 2.5L at 1.0atm pressure and the final volume is 5.0L at 0.5atm pressure. Using the first law of thermodynamics, the work done by the gas as it expands from the initial to final state is calculated to be 1.25J.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document describes a gas enclosed in a cylinder where the initial volume is 2.5L at 1.0atm pressure and the final volume is 5.0L at 0.5atm pressure. Using the first law of thermodynamics, the work done by the gas as it expands from the initial to final state is calculated to be 1.25J.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
10 views1 pageAssignment 1
Assignment 1
Uploaded by
loli XxxxThe document describes a gas enclosed in a cylinder where the initial volume is 2.5L at 1.0atm pressure and the final volume is 5.0L at 0.5atm pressure. Using the first law of thermodynamics, the work done by the gas as it expands from the initial to final state is calculated to be 1.25J.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
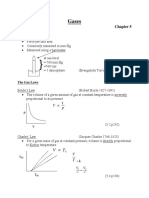
A gas is enclosed in a cylinder with a piston. The initial volume of the gas is 2.
5L, and the final volume is 5.0L. The
initial pressure of the gas is 1.0atm and the final pressure is 0.5atm. Using the first law of thermodynamics, calculate
the work done by the gas.
Solution: W = P(Vf - Vi) = (0.5 atm)(5.0L - 2.5L) = 1.25atm*L = 1.25J
You might also like
- Combined Gas Law & Dalton's Law (G4)Document37 pagesCombined Gas Law & Dalton's Law (G4)Kesziah CalambaNo ratings yet
- GASESDocument39 pagesGASESKarl Oliver Catabay Ricardo100% (1)
- Ideal Gas Law Problems and SolutionsDocument1 pageIdeal Gas Law Problems and SolutionsBasic Physics100% (1)
- 6 Gases PDFDocument70 pages6 Gases PDFRogerine RoyNo ratings yet
- GASESDocument101 pagesGASESdwyquishNo ratings yet
- Chapter 5 GasesDocument100 pagesChapter 5 GasesAhmed Qazi100% (1)
- Ideal Gas Law ExercisesDocument3 pagesIdeal Gas Law Exercisesloli XxxxNo ratings yet
- Assessment Daltons LawDocument12 pagesAssessment Daltons LawLance MonterolaNo ratings yet
- Chapter 5 GasesDocument74 pagesChapter 5 GasesReem HamadNo ratings yet
- Daltons Law of Partial PressuresDocument13 pagesDaltons Law of Partial PressuresKylene Grace IlaganNo ratings yet
- Chapter 5 GasesDocument100 pagesChapter 5 GasesFABIO DE LIMANo ratings yet
- MITCHIKDocument13 pagesMITCHIKMitchiko MondoyNo ratings yet
- Gases and Kinetic Molecular TheoryDocument71 pagesGases and Kinetic Molecular TheoryDebrah DebbieNo ratings yet
- Chapter 5 GasesDocument20 pagesChapter 5 GasesKevin MellizaNo ratings yet
- Chang Overby CH-5 HW PDFDocument38 pagesChang Overby CH-5 HW PDFRalph Evidente0% (1)
- AP CH 5 GasesDocument37 pagesAP CH 5 GasesyourstrulyrahulNo ratings yet
- Science Week 1 Chemistry q4Document9 pagesScience Week 1 Chemistry q4Mark Lenard BarawidanNo ratings yet
- Chapter 2 PensyarahDocument75 pagesChapter 2 PensyarahAdi BaddNo ratings yet
- Chapter 5 Gases PDFDocument49 pagesChapter 5 Gases PDFAbou WalidNo ratings yet
- Chapter 5 - The Gaseous StateDocument33 pagesChapter 5 - The Gaseous StateRashid EmoroniNo ratings yet
- Topic 1 - Gas Laws (Part 2)Document45 pagesTopic 1 - Gas Laws (Part 2)Joshua LaBordeNo ratings yet
- LO 5.3-The Ideal Gas Law and Its Applications: Chapter 6.4 + 6.5 in The TextbookDocument38 pagesLO 5.3-The Ideal Gas Law and Its Applications: Chapter 6.4 + 6.5 in The TextbookAmina AlmarzooqiNo ratings yet
- Lesson - Lec - KMT and Gas LawsDocument15 pagesLesson - Lec - KMT and Gas LawsVILLIE ALASNo ratings yet
- Lesson 2A Gas Laws 2Document22 pagesLesson 2A Gas Laws 2Desiree FranciscoNo ratings yet
- Boyle's LawDocument2 pagesBoyle's LawHinata CosaNo ratings yet
- Section 3-7Document32 pagesSection 3-7api-245255231No ratings yet
- Hand Out 4Document5 pagesHand Out 4Shawty YeshiNo ratings yet
- Chapter 5Document57 pagesChapter 5jenan.mizyedNo ratings yet
- Lecturer 2-FOEDocument14 pagesLecturer 2-FOEamr.120230006No ratings yet
- The Kinetic Molecular Theory: General Chemistry 1 Reviewer: 2nd QuarterDocument15 pagesThe Kinetic Molecular Theory: General Chemistry 1 Reviewer: 2nd QuarterJerome jeromeNo ratings yet
- Answer Key of Science ProblemDocument2 pagesAnswer Key of Science ProblemGrace Mary Tedlos BoocNo ratings yet
- AIR QUALITY AND POLLUTION (TKA 3301) LECTURE NOTES 4-Chemistry of Air Pollution N Ideal Gas LawDocument66 pagesAIR QUALITY AND POLLUTION (TKA 3301) LECTURE NOTES 4-Chemistry of Air Pollution N Ideal Gas Lawmamat88No ratings yet
- General Formula: P V P VDocument2 pagesGeneral Formula: P V P VJohn Rotrix TumanengNo ratings yet
- Chapter 5 CHEM110Document59 pagesChapter 5 CHEM110gracetetu102No ratings yet
- Module 5.2a: Gas Laws Part 1Document26 pagesModule 5.2a: Gas Laws Part 1Ryan PazonNo ratings yet
- GasesDocument41 pagesGasesJason BrozoNo ratings yet
- Chapter 5 PPTDocument42 pagesChapter 5 PPTSaikumar PNo ratings yet
- Daltons Law of Partial PressuresDocument50 pagesDaltons Law of Partial PressuresCassandra MhayNo ratings yet
- Gases: Behavior & PropertiesDocument35 pagesGases: Behavior & PropertiesAdelia SetianingsihNo ratings yet
- 10 Gases 2b PDFDocument10 pages10 Gases 2b PDFchewazableNo ratings yet
- Chapter 5 GasesDocument8 pagesChapter 5 GasessamNo ratings yet
- Properties of Gases: Rapidly and Collide With Each Other OftenDocument12 pagesProperties of Gases: Rapidly and Collide With Each Other OftenjohnloopsNo ratings yet
- Chapter 11 - Gas LawsDocument55 pagesChapter 11 - Gas Lawsjim tannerNo ratings yet
- Sorsogon National High School: Self-Directed Learning Activity Sheet in General Chemistry 1 (Las 8)Document4 pagesSorsogon National High School: Self-Directed Learning Activity Sheet in General Chemistry 1 (Las 8)Jorgia lianne UrbanoNo ratings yet
- Boyle's LawDocument14 pagesBoyle's LawRutchie Quillo Tuando100% (1)
- Behavior of Gases Module 1Document25 pagesBehavior of Gases Module 1Jericho GojocruzNo ratings yet
- Combined Gas LawsDocument7 pagesCombined Gas LawsGabriela UnoNo ratings yet
- Q4 Science10 Week1 LAS2Document1 pageQ4 Science10 Week1 LAS2AvaricioElPecadoNo ratings yet
- Gas LawsDocument24 pagesGas LawsJohn Louie NocheNo ratings yet
- CHEMISTRYDocument5 pagesCHEMISTRYLeila CruzNo ratings yet
- (Lec5) Properties of GasesDocument52 pages(Lec5) Properties of GasesdinurjNo ratings yet
- Chem 73 PS1 2017 PDFDocument3 pagesChem 73 PS1 2017 PDFImee Kassandra Estomo CachoNo ratings yet
- G10 4TH NotesDocument6 pagesG10 4TH Notesmarife gupaalNo ratings yet
- GasesDocument2 pagesGasesCarlos FrancoNo ratings yet
- Science10 Boyles LawDocument12 pagesScience10 Boyles LawEzekiel EsperanzateNo ratings yet
- Gas Laws PPT Combined and Avogadros LawDocument24 pagesGas Laws PPT Combined and Avogadros LawAngela Mae VillalunaNo ratings yet
- Gas Laws Review Sheet Answers RenoDocument2 pagesGas Laws Review Sheet Answers RenoERICA BURNSNo ratings yet
- Chem 181 Chemistry of GasesDocument15 pagesChem 181 Chemistry of GasesJoey PooleNo ratings yet
- Ideal Gas Law ExercisesDocument3 pagesIdeal Gas Law Exercisesloli XxxxNo ratings yet
- Energy Conservation ExercisesDocument6 pagesEnergy Conservation Exercisesloli XxxxNo ratings yet
- Chemical Quantities ExercisesDocument2 pagesChemical Quantities Exercisesloli XxxxNo ratings yet
- Chemical Molarity and Molality ExercisesDocument3 pagesChemical Molarity and Molality Exercisesloli XxxxNo ratings yet
- Psychrometric Chart ExercisesDocument1 pagePsychrometric Chart Exercisesloli XxxxNo ratings yet
- Thermodynamics Cycles ExercisesDocument3 pagesThermodynamics Cycles Exercisesloli XxxxNo ratings yet
- Engineering Thermodynamics - Open Systems ExercisesDocument1 pageEngineering Thermodynamics - Open Systems Exercisesloli XxxxNo ratings yet
- Psychrometry Exercises With SolutionsDocument1 pagePsychrometry Exercises With Solutionsloli XxxxNo ratings yet
- First Law of ThermodynamicsDocument2 pagesFirst Law of Thermodynamicsloli XxxxNo ratings yet