Professional Documents
Culture Documents
Dative Covalent Bonding
Dative Covalent Bonding
Uploaded by
Aathifa Thowfeek0 ratings0% found this document useful (0 votes)
3 views3 pagesBond energy refers to the energy required to break a covalent bond between two atoms, and is measured in kJ/mol. The stronger the bond, the higher the bond energy. Bond length is the distance between the nuclei of two covalently bonded atoms. Triple bonds are the shortest and strongest due to their high electron density, while increased electron-nuclei attraction results in shorter, stronger bonds. Dative bonding occurs when a lone pair of electrons from one atom forms a bond with an electron-deficient atom. Examples include the ammonium ion and aluminum chloride dimer.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentBond energy refers to the energy required to break a covalent bond between two atoms, and is measured in kJ/mol. The stronger the bond, the higher the bond energy. Bond length is the distance between the nuclei of two covalently bonded atoms. Triple bonds are the shortest and strongest due to their high electron density, while increased electron-nuclei attraction results in shorter, stronger bonds. Dative bonding occurs when a lone pair of electrons from one atom forms a bond with an electron-deficient atom. Examples include the ammonium ion and aluminum chloride dimer.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
3 views3 pagesDative Covalent Bonding
Dative Covalent Bonding
Uploaded by
Aathifa ThowfeekBond energy refers to the energy required to break a covalent bond between two atoms, and is measured in kJ/mol. The stronger the bond, the higher the bond energy. Bond length is the distance between the nuclei of two covalently bonded atoms. Triple bonds are the shortest and strongest due to their high electron density, while increased electron-nuclei attraction results in shorter, stronger bonds. Dative bonding occurs when a lone pair of electrons from one atom forms a bond with an electron-deficient atom. Examples include the ammonium ion and aluminum chloride dimer.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 3
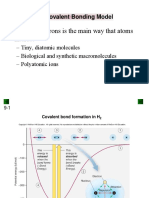
Covalent Bonding: Bond Energy & Length
Bond energy
The bond energy is the energy required to break one mole of a particular covalent bond in
the gaseous states
o Bond energy has units of kJ mol-1
The larger the bond energy, the stronger the covalent bond is
Bond length
The bond length is internuclear distance of two covalently bonded atoms
o It is the distance from the nucleus of one atom to another atom which forms the
covalent bond
The greater the forces of attraction between electrons and nuclei, the more the atoms are
pulled closer to each other
This decreases the bond length of a molecule and increases the strength of the covalent
bond
Triple bonds are the shortest and strongest covalent bonds due to the large electron density
between the nuclei of the two atoms
This increase the forces of attraction between the electrons and nuclei of the atoms
As a result of this, the atoms are pulled closer together causing a shorter bond length
The increased forces of attraction also means that the covalent bond is stronger
Dative Bonding: Definition & Examples
In simple covalent bonds the two atoms involved shares electrons
Some molecules have a lone pair of electrons that can be donated to form a bond with
an electron-deficient atom
o An electron-deficient atom is an atom that has an unfilled outer orbital
So both electrons are from the same atom
This type of bonding is called dative covalent bonding or coordinate bond
An example of a dative bond is in an ammonium ion
Aluminium chloride is also formed using dative covalent bonding
At high temperatures aluminium chloride can exist as a monomer (AlCl3)
o The molecule is electron-deficient and needs to electrons complete the aluminium
atom’s outer shell
At lower temperatures the two molecules of AlCl3 join together to form a dimer
(Al2Cl6)
o The molecules combine because lone pairs of electrons on two of the chlorine
atoms form two coordinate bonds with the aluminium atoms
You might also like
- Structure and Bonding IPADDocument4 pagesStructure and Bonding IPADLarissa RubarajNo ratings yet
- Chemical BondingDocument53 pagesChemical Bondingpream.s1323No ratings yet
- Bonding Essay: IFP Engineering and Science January IntakeDocument6 pagesBonding Essay: IFP Engineering and Science January IntakeGhassan MousaNo ratings yet
- Introduction To Chemical Bonding: Ionic Bond OR Electrovalent BondDocument12 pagesIntroduction To Chemical Bonding: Ionic Bond OR Electrovalent Bondanon_397573801No ratings yet
- Lecture-3-Chemical Bonds and Functional GroupsDocument64 pagesLecture-3-Chemical Bonds and Functional GroupsWiza MulengaNo ratings yet
- Chemical Bonding and Molecular Structure Class 11 Notes Chemistry Chapter 4Document17 pagesChemical Bonding and Molecular Structure Class 11 Notes Chemistry Chapter 411 A Prasann JamaleNo ratings yet
- Chemical BondingDocument83 pagesChemical BondingMd.Tanjim reza TurjoNo ratings yet
- Chemical BondingDocument7 pagesChemical BondingSanaa SamkoNo ratings yet
- 2023 Grade 11 Learner Support DocumentDocument131 pages2023 Grade 11 Learner Support DocumentanathinothandoNo ratings yet
- 11 Chemistry Handout Chapter 4Document17 pages11 Chemistry Handout Chapter 4Erreneo100% (1)
- What Is A Covalent Bond?Document4 pagesWhat Is A Covalent Bond?Gunjeet KaurNo ratings yet
- Chapter 4-Chemical BondingDocument1 pageChapter 4-Chemical Bondingsnigdhamishra0088No ratings yet
- Bond Order Bond Length: Picometers, PM Bond EnergyDocument1 pageBond Order Bond Length: Picometers, PM Bond EnergyHelen DichosonNo ratings yet
- Notes On Chemical Bonding and Molecular StructureDocument17 pagesNotes On Chemical Bonding and Molecular StructureDeepti KashyapNo ratings yet
- Chemical Bonding and Molecular StructureDocument12 pagesChemical Bonding and Molecular StructureM. MuvafficaNo ratings yet
- Material Chapter 2 Atomic Structure and BondingDocument19 pagesMaterial Chapter 2 Atomic Structure and BondingÇãłl Mê MęlkãNo ratings yet
- Islamic University, Kushtia-7003: Department of PharmacyDocument29 pagesIslamic University, Kushtia-7003: Department of PharmacyRayhanuzzaman ShazibNo ratings yet
- Class04 ChemistryG12 Notes and HomeworkDocument58 pagesClass04 ChemistryG12 Notes and HomeworkAndy Rei KouNo ratings yet
- Cape Chemistry Chapter 2 Structure and BondingDocument27 pagesCape Chemistry Chapter 2 Structure and Bondingchelsea AlexandriaNo ratings yet
- Chem1 3 - BondingDocument6 pagesChem1 3 - Bondingapi-247243068No ratings yet
- Chemical Bonding and Molecular Structure NotesDocument17 pagesChemical Bonding and Molecular Structure Notesarjunrkumar2024No ratings yet
- Chapter 2, Atkins Chemical Principles The Quest For InsightDocument6 pagesChapter 2, Atkins Chemical Principles The Quest For InsightericthecmhNo ratings yet
- Chemical BondingDocument10 pagesChemical BondingMalik MaazNo ratings yet
- Presented By:: Section: A Group No.: 4Document56 pagesPresented By:: Section: A Group No.: 4Bela GhummanNo ratings yet
- Chemical Bondind and Molecular StructureDocument33 pagesChemical Bondind and Molecular StructureSaadNo ratings yet
- Chemical Bonding 1Document20 pagesChemical Bonding 1ahmedNo ratings yet
- Bonding & Structure NotesDocument11 pagesBonding & Structure NotesKamran TajbakhshNo ratings yet
- Chemical Bonding: A MoleculeDocument88 pagesChemical Bonding: A MoleculeVrisanNo ratings yet
- Properties of BondsDocument36 pagesProperties of BondsPaulNo ratings yet
- Unit 3 - Chemical BondingDocument56 pagesUnit 3 - Chemical BondingAchini SheharaNo ratings yet
- 2 2 2 Bonding and StructureDocument7 pages2 2 2 Bonding and StructureifratsubhaNo ratings yet
- PHSC GR 11 REVISION DOCUMENT APRIL 2022 Collated Final Final PDFDocument66 pagesPHSC GR 11 REVISION DOCUMENT APRIL 2022 Collated Final Final PDFPhilNo ratings yet
- Chem Structure BondingDocument7 pagesChem Structure BondingJake blakeNo ratings yet
- 11 Chemistry-Chemical Bonding and Molecular Struture - NotesDocument23 pages11 Chemistry-Chemical Bonding and Molecular Struture - NotesSumit MahatoNo ratings yet
- BondingDocument3 pagesBondingNarjis FatimaNo ratings yet
- Chapter 4 Chemical BondingDocument45 pagesChapter 4 Chemical BondingRavinder singhNo ratings yet
- VND Openxmlformats-Officedocument PresentationmlDocument56 pagesVND Openxmlformats-Officedocument Presentationmlbala6927No ratings yet
- Assignment Chem 1Document6 pagesAssignment Chem 1laybah jawaydNo ratings yet
- Chapter Two Chemical BondinG EDDocument77 pagesChapter Two Chemical BondinG EDnasec38602No ratings yet
- Covalent BondsDocument12 pagesCovalent Bondsjennifer ansahNo ratings yet
- Chemical BondDocument8 pagesChemical BondRohan lallNo ratings yet
- Chemical Bonding Class 11Document18 pagesChemical Bonding Class 11bansarigadhvi23No ratings yet
- By AdithyaDocument35 pagesBy AdithyaA SQUARE GAMING DEVIL L7ADILHYANo ratings yet
- Covalent CompoundsDocument12 pagesCovalent CompoundsgrazianirNo ratings yet
- Chemical BondingDocument69 pagesChemical Bondingirenerielo19No ratings yet
- Atomic Structure and BondingDocument15 pagesAtomic Structure and BondingPaidamoyo MunhengaNo ratings yet
- Covalent Bonding Lesson - CompressedDocument42 pagesCovalent Bonding Lesson - CompressedkrischelleNo ratings yet
- CHEMICAL BONDINGgDocument63 pagesCHEMICAL BONDINGgTabiku Sultana OrpaNo ratings yet
- Chemical BondingDocument94 pagesChemical BondingGagandeep WadhawanNo ratings yet
- Class 10th Chemistry NotesDocument4 pagesClass 10th Chemistry NotesRehan KhanNo ratings yet
- Tej and VijendraDocument8 pagesTej and Vijendrajnvnavsari10No ratings yet
- Chapter 6 Shapes of Molecules and Intermolecular ForcesDocument9 pagesChapter 6 Shapes of Molecules and Intermolecular Forcesnoreen doraniNo ratings yet
- Class XI Chemical Bonding and Molecular Structure NotesDocument13 pagesClass XI Chemical Bonding and Molecular Structure NoteseasaNo ratings yet
- 4.chemical Bonding and Molecular Structure - 2016Document10 pages4.chemical Bonding and Molecular Structure - 2016Karanvir Singh 6No ratings yet
- Chemical Bonding 1Document81 pagesChemical Bonding 1RIMMY AUGUSTINE 2138110No ratings yet
- AQA Chemistry A-Level: 3.1.3: Bonding and StructuresDocument10 pagesAQA Chemistry A-Level: 3.1.3: Bonding and StructuresAshleigh NcubeNo ratings yet
- Chemistry Module TwoDocument111 pagesChemistry Module TwoGreg YianniNo ratings yet
- Chemical CombinationDocument4 pagesChemical CombinationGodspower OgbonnayaNo ratings yet
- Concept Review With Key TermsDocument4 pagesConcept Review With Key TermsWaqar AhmedNo ratings yet
- Chemical Bonding: Understanding The Forces that Hold Molecules Together.From EverandChemical Bonding: Understanding The Forces that Hold Molecules Together.No ratings yet
- Chemistry - GR 08 - Nov 2023Document9 pagesChemistry - GR 08 - Nov 2023Aathifa ThowfeekNo ratings yet
- 2Document1 page2Aathifa ThowfeekNo ratings yet
- Blood Circulatory SystemDocument5 pagesBlood Circulatory SystemAathifa ThowfeekNo ratings yet
- Ethical Guidelines For The Treatment of AnimalsDocument1 pageEthical Guidelines For The Treatment of AnimalsAathifa ThowfeekNo ratings yet
- Fish FarmingDocument2 pagesFish FarmingAathifa ThowfeekNo ratings yet
- IUPAC NomenclatureDocument5 pagesIUPAC NomenclatureAathifa ThowfeekNo ratings yet
- Fuel ProductiomDocument3 pagesFuel ProductiomAathifa ThowfeekNo ratings yet
- Ionisation EnergyDocument6 pagesIonisation EnergyAathifa ThowfeekNo ratings yet
- The Vascular Structure of PlantsDocument7 pagesThe Vascular Structure of PlantsAathifa ThowfeekNo ratings yet
- Incomplete CombustionDocument3 pagesIncomplete CombustionAathifa ThowfeekNo ratings yet
- Mass SpectrometryDocument2 pagesMass SpectrometryAathifa ThowfeekNo ratings yet
- Aspire Business School-Term Exam1: BiologyDocument20 pagesAspire Business School-Term Exam1: BiologyAathifa ThowfeekNo ratings yet
- 7 The Diagram Shows The Born-Haber Cycle For Magnesium ChlorideDocument6 pages7 The Diagram Shows The Born-Haber Cycle For Magnesium ChlorideAathifa ThowfeekNo ratings yet
- Crude OilDocument8 pagesCrude OilAathifa ThowfeekNo ratings yet
- Science ExamDocument11 pagesScience ExamAathifa ThowfeekNo ratings yet