Professional Documents
Culture Documents
Asrjc QP
Uploaded by
Lorraine HoonOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Asrjc QP
Uploaded by
Lorraine HoonCopyright:
Available Formats
ANDERSON SERANGOON JUNIOR COLLEGE
2021 JC 2 PRELIMINARY EXAMINATION
NAME:_________________________________ ( ) CLASS: 21 /____
CHEMISTRY 9729/02
Paper 2 Structured Questions 14 September 2021
2 hours
Candidates answer on the Question Paper.
Additional Materials: Data Booklet
READ THESE INSTRUCTIONS FIRST
Write your name, class and register number in the spaces at the top of this page.
Write in dark blue or black pen.
You may use an HB pencil for any diagrams or graphs.
Do not use staples, paper clips, glue or correction fluid.
Answer all questions in the spaces provided on the Question Paper.
The use of an approved scientific calculator is expected, where appropriate.
A Data Booklet is provided.
The number of marks is given in brackets [ ] at the end of each question or part question.
For Examiner’s Use
1 /21
2 /10
3 /13
Paper 2
4 /10
5 /11
6 /10
Total /75
This document consists of 23 printed pages and 1 blank page.
ASRJC JC2 PRELIMS 2021 9729/02/H2 [Turn over
2
Answer all the questions in the spaces provided.
1 (a) Describe and explain the trend in electronegativity of elements across Period 3 from sodium
to chlorine.
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
……………………………………………………………………………………………………….[2]
(b) Some ionic radii are listed in the Data Booklet.
(i) Explain the trend in ionic radius down Group 2.
……………………………………………………………………………………………………
……………………………………………………………………………………………………
……………………………………………………………………………………………………
……………………………………………………………………………………………………
…………………………………………………………………………………………………[2]
(ii) Explain the differences between the ionic radii of P3–, Cl– and Ca2+.
……………………………………………………………………………………………………
……………………………………………………………………………………………………
……………………………………………………………………………………………………
……………………………………………………………………………………………………
…………………………………………………………………………………………………[1]
(iii) Hence, suggest a value for the ionic radius of a potassium ion, K+.
…………………………………………………………………………………………………[1]
ASRJC JC2 PRELIMS 2021 9729/02/H2
3
(iv) Explain the difference in size between the radius of the potassium ion and the radius of
a potassium atom.
……………………………………………………………………………………………………
……………………………………………………………………………………………………
…………………………………………………………………………………………………[1]
(c) Anodisation of aluminium is a process which coats an oxide layer on aluminium objects.
(i) Draw a labelled diagram of the electrolysis cell used to anodise a small piece of
aluminium object. Include details of the cathode, anode and electrolyte.
[1]
(ii) Complete Table 1.1 to show the type of reaction occurring, with the relevant
half–equations, during the anodisation of the aluminium object.
Table 1.1
type of reaction
half–equation(s)
occurring
anode
cathode
[2]
ASRJC JC2 PRELIMS 2021 9729/02/H2 [Turn over
4
(d) The molecules of alcohol P, C7H16O, are optically active and does not react with hot, acidified
Na2Cr2O7(aq).
On treatment with Al2O3, P produces a mixture of four different isomeric alkenes with the
formula C7H14, only two of which are cis–trans isomers of each other.
Suggest the structural formula of compound P and the four alkenes.
[4]
ASRJC JC2 PRELIMS 2021 9729/02/H2
5
Question 1 continues on the next page.
ASRJC JC2 PRELIMS 2021 9729/02/H2 [Turn over
6
(e) The following equilibrium exists in a sample of aluminium chloride vapour.
Al2Cl6(g) ⇌ 2AlCl3(g)
(i) Draw a dot–and–cross diagram of the Al2Cl6 molecule, including its co–ordinate bonds.
[1]
When 1.50 g of aluminium chloride was introduced into an evacuated flask of 250 cm 3
capacity and heated to 500 K, the pressure inside the flask rose to 1.16 x 105 Pa.
(ii) Assuming the gaseous mixture behaves ideally, calculate the average Mr of the mixture.
Give your answer to four significant figures.
[2]
(iii) Using the following relationships, calculate the mole fraction of Al2Cl6, x and the mole
fraction of AlCl3, y, in the mixture.
x+y=1
average Mr = 267x + 133.5y
[1]
ASRJC JC2 PRELIMS 2021 9729/02/H2
7
(iv) Hence calculate the partial pressures of Al2Cl6 and AlCl3 in this mixture.
[1]
(v) Write an expression for Kp for the reaction, and calculate its value. Include units in your
answer.
[2]
[Total: 21]
ASRJC JC2 PRELIMS 2021 9729/02/H2 [Turn over
8
2 (a) When Group 2 iodates(V), M(IO3)2, is heated, it behaves in a similar way to the Group 2
carbonates. Upon heating, it decomposes as shown.
2M(IO3)2(s) 2MO(s) + 2I2(g) + 5O2(g) (where M is a Group 2 metal)
(i) Using your knowledge of Group 2 carbonates, suggest and explain the trend in thermal
stabilities of the Group 2 iodate(V).
…………………………………………………………………………………………………….
…………………………………………………………………………………………………….
…………………………………………………………………………………………………….
…………………………………………………………………………………………………….
…...…………………………………………………………………………………………….[2]
X, Y and Z are Group 2 metals (Mg to Ba, not necessarily in that order).
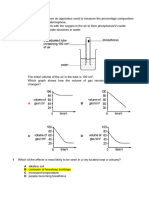
X(IO3)2, Y(IO3)2 and Z(IO3)2 are Group 2 iodates(V). The three graphs in Fig. 2.1 show the
change in mass when 2.00 g each of X(IO3)2, Y(IO3)2 and Z(IO3)2 were heated separately at
a temperature T oC.
Fig. 2.1
(ii) With reference to the information from Fig. 2.1, show, by calculations, that none of the
above iodate(V) samples contains Mg(IO3)2.
[2]
ASRJC JC2 PRELIMS 2021 9729/02/H2
9
(iii) Hence, suggest the identities of the three iodates(V).
[No calculation is required.]
iodates(V) X(IO3)2 Y(IO3)2 Z(IO3)2
identity
[1]
(b) When a salt such as a Group 2 sulfate dissolves in water, the lattice energy must be
overcome.
(i) How will the magnitude of the lattice energy of Group 2 sulfates change from MgSO4
to BaSO4?
…………………………………………………………………………………………………....
………………………………………………………………………………………………....[1]
(ii) Suggest a reason for this trend.
…………………………………………………………………………………………………….
…………………………………………………………………………………………………….
…………………………………………………………………………………………………….
…………………………………………………………………………………………………….
……………………………………………………………………………………………....…[1]
(iii) Draw a simple diagram to show how a water molecule can be attached to a magnesium
cation, and to a sulfate anion. Show the displayed structure of the sulfate anion in your
answer and label each diagram to show the type of interaction involved.
Mg2+ cation SO42– anion
[3]
[Total: 10]
ASRJC JC2 PRELIMS 2021 9729/02/H2 [Turn over
10
Question 3 starts on the next page.
ASRJC JC2 PRELIMS 2021 9729/02/H2
11
3 Trisoxazoline are organic molecules that can function as ligands. Despite their huge molecular
structure, they are able to form stable complexes with metals. Metal complexes with trisoxazoline,
such as copper(II)-trisoxazoline, are commonly used as catalyst in organic synthesis.
(a) With the aid of a Boltzmann distribution, explain how a catalyst increases the rate of reaction.
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
……………………………………………………………………………………………………….[3]
ASRJC JC2 PRELIMS 2021 9729/02/H2 [Turn over
12
(b) Fig. 3.1 illustrates the synthesis of trisoxazoline.
OH OH Cl
O O O O
N N N
OH step I OH step II Cl
Cl O
HO HO O
step III
H O
N N
O
O O O O
organic base
O O N H
N
N step IV N
O
N N O
O
H O
trisoxazoline
Fig. 3.1
(i) State the types of reaction that occur during each of the steps I and II.
step I: …………………………………………………………………………………….
step II: …………………………………………………………………………………….
[2]
(ii) Suggest, with reason, the pH of the solution when the resultant product from
step II is dissolved in water.
……………………………………………………………………………………………………
…………………………………………………………………………………………………[1]
(iii) Suggest the reagents and conditions required for steps II and III.
step II: …………………………………………………………………………………….
step III: …………………………………………………………………………………….
[2]
ASRJC JC2 PRELIMS 2021 9729/02/H2
13
(iv) Compound P in Fig. 3.1 can be synthesised from ethene via a two–step reaction
scheme shown below.
step a step b
ethene 2–chloroethanol P
Suggest the reagents required for each step.
step a: …………………………………………………………………………………….
step b: …………………………………………………………………………………….
[2]
(c) Other than copper(II) ions, it was found that copper(I) ions are also able to form complexes
with trisoxazoline.
Complete the full electronic configuration of copper(I) ions.
copper(I) ion: 1s2 ……………………………………………………………………………… [1]
(d) Copper is in increasing demand for use in electric vehicles, consumer electronics and other
energy efficient targets. Most current copper extraction processes burn sulfide minerals in air
which produces sulfur dioxide which is harmful to the environment.
Describe and explain with the aid of suitable equations, the role of NO 2 in the oxidation of
atmospheric sulfur dioxide.
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
……………………………………………………………………………………………………….[2]
[Total: 13]
ASRJC JC2 PRELIMS 2021 9729/02/H2 [Turn over
14
4 (a) A 2.00 g sample of an organic substance Z containing only C, H and O was burned
completely. The only combustion products were 2.90 g of carbon dioxide and 1.20 g of water.
(i) Define the term relative molecular mass.
……………………………………………………………………………………………………
…………………………………………………………………………………………………[1]
(ii) Given that the relative molecular mass of the organic substance Z is 90.0, show that
the molecular formula is C3H6O3.
[2]
(iii) Draw two constitutional isomers of Z which can react with sodium carbonate.
[2]
ASRJC JC2 PRELIMS 2021 9729/02/H2
15
(b) Complete the reaction scheme in Fig. 4.1 to show how compound E could be synthesised
from ethylbenzene in three steps using suitable reagents.
Show the structures of the intermediate compounds and state the reagents and conditions
for each step.
Step 1 Step 2
intermediate intermediate
compound compound
Step 3
OH
NO2
E
Fig. 4.1
Step 1: ……………………………………………………………………………………….….
Step 2: …………………………………………………………………………………………..
Step 3: …………………………………………………………………………………………..
[5]
[Total: 10]
ASRJC JC2 PRELIMS 2021 9729/02/H2 [Turn over
16
Question 5 starts on the next page.
ASRJC JC2 PRELIMS 2021 9729/02/H2
17
5 This question is about the chemistry of compounds containing halogen.
(a) Bromoalkane can undergo two different mechanisms for nucleophilic substitution − SN1 and
SN2.
Experiments were conducted in two different set–ups to measure the relative rates of
nucleophilic substitution for three bromoalkanes. Set–up 1 and 2 use different nucleophiles.
Table 5.1 summarises the relative rates of the three bromoalkanes in the experiments.
Table 5.1
bromoalkane CH3CH2Br (CH3)2CHBr (CH3)3CBr
relative rate in set–up 1 4 x 10–2 1 4 x 106
relative rate in set–up 2 30 1 5 x 10–5
(i) Predict the predominant mechanism for set–up 2. Explain your answer.
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
……………………………………………………………………………………………………….[2]
(ii) Explain the relative rate of (CH3)3CBr in set–up 1.
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
……………………………………………………………………………………………………….[1]
ASRJC JC2 PRELIMS 2021 9729/02/H2 [Turn over
18
(b) 2–iodobutane can be readily converted into 2–aminobutane using ethanolic ammonia.
CH3CHICH2CH3 + 2NH3 CH3CH(NH2)CH2CH3 + NH4I
In order to determine the rate equation for this reaction, an investigation was carried out at a
constant temperature. Equal volume of 0.20 mol dm−3 2–iodobutane and 4.00 mol dm−3
ethanolic ammonia were mixed. At suitable time intervals, 10 cm3 of the reaction mixture was
pipetted into a conical flask and quenched.
Chlorine gas was bubbled into the aliquot and excess chlorine gas was subsequently
removed. Iodine is liberated by the following reaction.
Cl2 + 2I− 2Cl− + I2
The iodine was then titrated with 0.0250 mol dm−3 sodium thiosulfate in the presence of an
indicator.
I2 + 2S2O32− 2I− + S4O62−
Fig. 5.1 shows the volume of sodium thiosulfate used against time for this experiment.
40
35
30
Volume of S2O32− / cm3
25
20
15
10
0
0 5 10 15 20 25 30 35
time / s
Fig. 5.1
ASRJC JC2 PRELIMS 2021 9729/02/H2
19
(i) Prove that the volume of S2O32– required when the reaction between 2–iodobutane and
ethanolic ammonia goes to completion in this investigation is 40 cm3.
[1]
(ii) Hence, use Fig. 5.1 to deduce the order of reaction with respect to 2–iodobutane.
order = .……………………………………[2]
ASRJC JC2 PRELIMS 2021 9729/02/H2 [Turn over
20
(iii) The concentration of ethanolic ammonia is halved and a new series of experiments was
carried out at the same temperature.
It is known that that the order of reaction with respect to ethanolic ammonia is one.
Suggest how the gradient at each point will change when a similar graph like the one
in Fig. 5.1 was plotted.
……………………………………………………………………………………………………
……………………………………………………………………………………………………
…………………………………………………………………………………………………[1]
(iv) Hence, construct the rate equation for the reaction between 2–iodobutane and ethanolic
ammonia.
…………………………………………………………………………………………………[1]
(v) Calculate the value of the rate constant for the reaction between equal volumes of
0.20 mol dm–3 2–iodobutane and 4.00 mol dm−3 ethanolic ammonia and state its units.
value of rate constant = ……………………………………
units = ……………………………………
[2]
ASRJC JC2 PRELIMS 2021 9729/02/H2
21
(c) Iodide ions, I−, can react with peroxodisulfate, S2O82−.
S2O82− + 2I− 2SO42− + I2
A student wanted to investigate the effect of changes in volume used on the rate of this
reaction.
Table 5.2 shows the results obtained when a series of experiments was carried out using
different volumes of the two reagents, each solution being made up to the same total volume
with water, where necessary.
Table 5.2
volume of KI volume of Na2S2O8 volume of water initial rate /
experiment
/ cm3 / cm3 / cm3 mol dm–3 s–1
1 10 20 10 0.0200
2 5 20 15 0.0100
3 30 10 0 0.0303
Given that the order of reaction with respect to I− is 1, determine the order of reaction with
respect to S2O82–.
order = ……………………………………[1]
[Total: 11]
ASRJC JC2 PRELIMS 2021 9729/02/H2 [Turn over
22
6 (a) Ammonia can act as a weak base.
NH3(aq) + H2O(l) ⇌ NH4+(aq) + OH–(aq) pKb = 4.75
(NH4)2SO4 is a weak acid. Calculate the pH of 0.10 mol dm–3 (NH4)2SO4(aq).
[2]
(b) A buffer solution with a pH 8.90 is made by adding 150 cm3 of solution B containing
ammonium chloride to 100 cm3 of 0.0200 mol dm–3 aqueous NH3.
Calculate the concentration of ammonium chloride in solution B.
[3]
(c) Write equations to explain how the NH3(aq) /NH4+(aq) buffer system helps to maintain the pH.
…………………………………………………………………………………………………….……
…………………………………………………………………………………………………….……
………………………………………………………………………………………….……………[2]
ASRJC JC2 PRELIMS 2021 9729/02/H2
23
(d) Malaria is a serious and sometimes fatal mosquito–borne disease. Primaquine is an
antimalaria drug used to prevent relapse of malaria infections.
b
a NH2
HN
N
primaquine
Two of the pKb values associated with primaquine are 4.1 and 9.8.
(i) Using this information, write the pKb values for the nitrogen–containing groups
a and b in primaquine.
nitrogen–containing group a group b
pKb
[1]
(ii) Suggest an explanation for your assignment of the pKb values in (d)(i).
……………………………………………………………………………………………………
……………………………………………………………………………………………………
……………………………………………………………………………………………………
……………………………………………………………………………………………………
……………………………………………………………………………………………………
……………………………………………………………………………………………………
…………………………………………………………………………………………………[2]
[Total: 10]
ASRJC JC2 PRELIMS 2021 9729/02/H2
24
BLANK PAGE
ASRJC JC2 PRELIMS 2021 9729/02/H2
You might also like
- Anderson Serangoon Junior College: 2021 JC 2 Mid Year Common TestDocument24 pagesAnderson Serangoon Junior College: 2021 JC 2 Mid Year Common TestpianorificationNo ratings yet
- 2021 JC1 H2 Promo Section B QNDocument12 pages2021 JC1 H2 Promo Section B QNFelysia DianniNo ratings yet
- ACJC Promo Section B, C - D QP (1.5hr) (2019 H2 Chem)Document16 pagesACJC Promo Section B, C - D QP (1.5hr) (2019 H2 Chem)Seon HoganNo ratings yet
- 2021 H2 JC1 Promo Section C QnsDocument16 pages2021 H2 JC1 Promo Section C QnsFelysia DianniNo ratings yet
- 2019 Jc1 Yee - Section B & C (QP)Document25 pages2019 Jc1 Yee - Section B & C (QP)AKASH SUBRAMANIAN 22S207No ratings yet
- A2 CHM Sol 05 Acid and Base WSDocument28 pagesA2 CHM Sol 05 Acid and Base WSnsNo ratings yet
- 2012 A Level Answers P1 and P2 Compiled FinalDocument12 pages2012 A Level Answers P1 and P2 Compiled FinalWesley TanNo ratings yet
- 2011 H2 Chemistry Paper 1 Suggested SolutionsDocument18 pages2011 H2 Chemistry Paper 1 Suggested SolutionsLee Jun HuiNo ratings yet
- TJC Chemistry H2 Y1 2009Document21 pagesTJC Chemistry H2 Y1 2009OccamsRazor100% (1)
- 2015 Promo - Section ADocument9 pages2015 Promo - Section AMelissa0% (1)
- 2020 ACJC Paper 4 Qns PDFDocument18 pages2020 ACJC Paper 4 Qns PDFchuasioklengNo ratings yet
- NYJC 2009 Prelim H2 P2 QuestionDocument14 pagesNYJC 2009 Prelim H2 P2 QuestioncjcsucksNo ratings yet
- 2019 JC1 H2 Math Term 2 Revision Test (Questions)Document1 page2019 JC1 H2 Math Term 2 Revision Test (Questions)Timothy HandokoNo ratings yet
- Chemical Equilibria Tutorial With AnsDocument6 pagesChemical Equilibria Tutorial With AnsDomNo ratings yet
- VJC 2007Document14 pagesVJC 2007sswee_1No ratings yet
- Answers - H2 Topical Chemistry 2014Document99 pagesAnswers - H2 Topical Chemistry 2014Ruel Arila Jr.No ratings yet
- HCI Chem H2 Paper 1 Question PaperDocument17 pagesHCI Chem H2 Paper 1 Question PaperonnoezNo ratings yet
- Nyjc - 2007 Jc1 h2 Promo p3 - AnswerDocument4 pagesNyjc - 2007 Jc1 h2 Promo p3 - AnswerSudibyo GunawanNo ratings yet
- 2019 JC2 H1 Economics Prelim River Valley High School AnswerDocument15 pages2019 JC2 H1 Economics Prelim River Valley High School AnswerTimothy HandokoNo ratings yet
- 4047 Specimen Paper 2 SolutionsDocument16 pages4047 Specimen Paper 2 SolutionsLester LimNo ratings yet
- 2015 PJC Prelim Paper 1 SolutionsDocument15 pages2015 PJC Prelim Paper 1 SolutionsnasyrahNo ratings yet
- Paper 2 Nov 2006Document6 pagesPaper 2 Nov 2006MSHNo ratings yet
- t2 Chem Revision Ex 15 MSDocument29 pagest2 Chem Revision Ex 15 MSNicholas Ow100% (1)
- ACJC 2014 H2 Math JC2 Supp QP PaperDocument7 pagesACJC 2014 H2 Math JC2 Supp QP PaperRaymondZhang100% (1)
- Chemistry HL Paper 2 TZ1Document36 pagesChemistry HL Paper 2 TZ1Hasnatul Khaira100% (1)
- Chemistry Paper 1 HL-Nov2017 PDFDocument17 pagesChemistry Paper 1 HL-Nov2017 PDFIrfan zameerNo ratings yet
- Victoria Junior College JC 2 Preliminary Examination 2017Document69 pagesVictoria Junior College JC 2 Preliminary Examination 2017Emmanuel Elijah ErNo ratings yet
- 2006 JC 1 H2 JCT & Promo - Differential EquationsDocument3 pages2006 JC 1 H2 JCT & Promo - Differential EquationsOccamsRazorNo ratings yet
- Sajc 2010 Prelim Math p1 SolnDocument10 pagesSajc 2010 Prelim Math p1 SolnAh XiuNo ratings yet
- 2022 Promotional Examination Revision Package: Learn DODocument118 pages2022 Promotional Examination Revision Package: Learn DOVincentNo ratings yet
- Alkane Alkene QuestionsDocument10 pagesAlkane Alkene QuestionsormattNo ratings yet
- 2009 Nov H2 Chemistry Paper 2Document6 pages2009 Nov H2 Chemistry Paper 2nncy_rox3478565No ratings yet
- MJC 2010 H2 Physics Prelim Paper 3xDocument21 pagesMJC 2010 H2 Physics Prelim Paper 3xcjcsucksNo ratings yet
- 4 E5 NPhy Prelim 2 MSrev V2Document7 pages4 E5 NPhy Prelim 2 MSrev V2Yee Kai TanNo ratings yet
- ASR 2020 J2Prelim H2Chem P4 QP PDFDocument20 pagesASR 2020 J2Prelim H2Chem P4 QP PDFchuasioklengNo ratings yet
- JC1 and JC2 H2 Physics 2015 Topical Revision (DHS)Document160 pagesJC1 and JC2 H2 Physics 2015 Topical Revision (DHS)tiffanyNo ratings yet
- JC H2 Chemistry Prelim PapersDocument13 pagesJC H2 Chemistry Prelim Paperschong56No ratings yet
- A Level H2 Paper 2Document6 pagesA Level H2 Paper 2newtonian_physics100% (1)
- Energetics - SL - 01: (Total 1 Mark)Document20 pagesEnergetics - SL - 01: (Total 1 Mark)Abhinaya PolakaNo ratings yet
- E MathDocument858 pagesE MathDamien SeowNo ratings yet
- A2 CHM 01 Energetics NotesDocument16 pagesA2 CHM 01 Energetics NotesMuhammad Saif AmirNo ratings yet
- 4047 SP 01Document6 pages4047 SP 01MaverickNo ratings yet
- 2021 JC2 Prelim H1 Chemistry Paper 1 QPDocument12 pages2021 JC2 Prelim H1 Chemistry Paper 1 QPShengxin PanNo ratings yet
- Atomic Structure and Periodic Table Mark SchemeDocument5 pagesAtomic Structure and Periodic Table Mark SchemeDiyaNo ratings yet
- GCE 5118 Nov08 P3Document16 pagesGCE 5118 Nov08 P3Hao TanNo ratings yet
- Moles P2Document6 pagesMoles P2ImranMalikNo ratings yet
- 2012 CJC CH h2 p2 PromoDocument12 pages2012 CJC CH h2 p2 PromoDaniel ChuNo ratings yet
- 05 Acid Base and Buffer WS 2021Document37 pages05 Acid Base and Buffer WS 2021VayaNo ratings yet
- 4E 2009 ZhongHua Prelim 2 Maths Paper 1Document18 pages4E 2009 ZhongHua Prelim 2 Maths Paper 1ZeneonNo ratings yet
- Topic 16 Past Paper Questions 2011Document15 pagesTopic 16 Past Paper Questions 2011nadia sykesNo ratings yet
- TMJC 2020 Mye j2Document15 pagesTMJC 2020 Mye j2toh tim lamNo ratings yet
- Chemistry Paper 1Document17 pagesChemistry Paper 1printdaddyNo ratings yet
- 2019 H2 Chemistry Paper 4 (Ans)Document7 pages2019 H2 Chemistry Paper 4 (Ans)Justin GohNo ratings yet
- Air and AtmosphereDocument12 pagesAir and Atmospherebob leowNo ratings yet
- 4016 Mathematics Topic 1: Numbers and AlgebraDocument7 pages4016 Mathematics Topic 1: Numbers and AlgebraMohammad AshfaqNo ratings yet
- Alkali Metals PPQDocument6 pagesAlkali Metals PPQ张米No ratings yet
- 2019 Jc1 Myct H2chem Paper 2 QPDocument15 pages2019 Jc1 Myct H2chem Paper 2 QPcolNo ratings yet
- J1 Promos 2015 Paper 2Document13 pagesJ1 Promos 2015 Paper 2aliciaNo ratings yet
- 0620 w17 QP 42Document16 pages0620 w17 QP 42gauthamNo ratings yet
- ME:5160 (58:160) Intermediate Mechanics of Fluids Fall 2021 - HW6 SolutionDocument6 pagesME:5160 (58:160) Intermediate Mechanics of Fluids Fall 2021 - HW6 SolutionDiki AgungNo ratings yet
- BNS-TCC Basler Time CurvesDocument8 pagesBNS-TCC Basler Time CurvesFelipeMoriNo ratings yet
- Chapter 8 - SynchronousDocument12 pagesChapter 8 - SynchronousLin ChongNo ratings yet
- A Clinical Study of Space Closure With Nickel-Titanium Closed Coil Springs and An Elastic ModuleDocument7 pagesA Clinical Study of Space Closure With Nickel-Titanium Closed Coil Springs and An Elastic ModuleValery Valer JaureguiNo ratings yet
- AISF302x en PDFDocument270 pagesAISF302x en PDFvsimongNo ratings yet
- The Performance and Service Life of Wire Ropes Under Deep Koepe and Drum Winders Conditions - Laboratory SimulationDocument9 pagesThe Performance and Service Life of Wire Ropes Under Deep Koepe and Drum Winders Conditions - Laboratory SimulationRicardo Ignacio Moreno MendezNo ratings yet
- MSC Aerospace Engineering PresentationDocument14 pagesMSC Aerospace Engineering PresentationMahmud Al HassanNo ratings yet
- Physical Science Module 3Document22 pagesPhysical Science Module 3Florence-j Pelayo Tupaz100% (1)
- Ashirwad Carbonics (India) Private Limited: Co2 Recovery Plant Technical LiteratureDocument5 pagesAshirwad Carbonics (India) Private Limited: Co2 Recovery Plant Technical LiteratureRaul BautistaNo ratings yet
- Iso 10724 1 1998Document11 pagesIso 10724 1 1998rtsultanNo ratings yet
- SV1-10-4/4M/4R: - Solenoid ValveDocument2 pagesSV1-10-4/4M/4R: - Solenoid ValveCORTOCIRCUITANTENo ratings yet
- Service Manual Repair Parts: Size 03 To 20 Posidyne Clutch/Brake DrivesDocument45 pagesService Manual Repair Parts: Size 03 To 20 Posidyne Clutch/Brake DrivesAnibal RodriguezNo ratings yet
- Door SheetDocument9 pagesDoor SheetAnilkumarNo ratings yet
- Horiba ABX Micros 60 - Technical Manual 2 PDFDocument205 pagesHoriba ABX Micros 60 - Technical Manual 2 PDFAniela RosalesNo ratings yet
- Python Lab 1Document4 pagesPython Lab 1vaskoreNo ratings yet
- Activate 1 BiologyDocument120 pagesActivate 1 BiologyMarina Belloni100% (1)
- Biochem Laboratory ReviewerDocument6 pagesBiochem Laboratory Reviewer202370092No ratings yet
- Vastu ShastraDocument36 pagesVastu ShastraAnjanaya Lamani0% (1)
- Curl and DivDocument3 pagesCurl and DivgjdapromiseNo ratings yet
- CTR Manufacturing Develops 2 Tap Changer Transformers With FR3 Fluid 1Document5 pagesCTR Manufacturing Develops 2 Tap Changer Transformers With FR3 Fluid 1SM KNo ratings yet
- Quantum Optical Memory For Entanglement DistributionDocument22 pagesQuantum Optical Memory For Entanglement DistributionJay-R Notorio PallegaNo ratings yet
- C Atomic StructureDocument34 pagesC Atomic StructureMarshmalloowNo ratings yet
- Unit 1Document57 pagesUnit 121ELB370MOHAMMAD AREEB HASAN KHANNo ratings yet
- 02-Continental Drift TheoryDocument22 pages02-Continental Drift Theoryapi-242405009No ratings yet
- EMV Multi Cable Transit Modular System (EMC-System) : PDFDocument7 pagesEMV Multi Cable Transit Modular System (EMC-System) : PDFbakien-canNo ratings yet
- Toro Wheelhorse Demystification Electical Wiring Diagrams For All WheelHorse TractorsDocument546 pagesToro Wheelhorse Demystification Electical Wiring Diagrams For All WheelHorse TractorsKevins Small Engine and Tractor Service69% (32)
- PDS Eden Piping PDFDocument140 pagesPDS Eden Piping PDFshaonaaNo ratings yet
- Availability Simulation - IsographDocument1 pageAvailability Simulation - IsographsaospieNo ratings yet
- Peter and Wolf Resource Guide - Boston Philharmonic OrchestraDocument32 pagesPeter and Wolf Resource Guide - Boston Philharmonic OrchestraEmily Sharratt100% (5)
- Xenon LampDocument25 pagesXenon LampJamaica Peñaloza NatuelNo ratings yet