Professional Documents
Culture Documents
Chem Notes
Uploaded by
Yu YuCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chem Notes
Uploaded by
Yu YuCopyright:
Available Formats
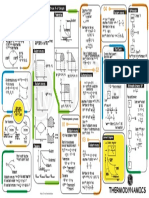
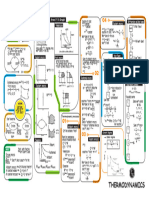
1 1
& ΔΔ
Process W
heatflow into or internal
work done change in
outof system energy
ene w
naubenCT.corhi
W 0 Δu a
Isochoric
=
ΔV 0 =
T & then PR ΔΔ R
=
I=E
Volume is constant when
volume is constant
n(pXT w/
N PAV E
Isobaric = a = 50 a = -
O
rest
molar heatcapacitypressure
ΔP 0 of
E=
=
<pressure is constant) T ↑ then Up. .I=
Isothermal W& =
& y=
Δu 0 =
ΔT 0 M nRT log []
] :PRX*=P2X
=
=
Ctemperature is constant)
& nRT
=
log [
W nRT
=
log [f]
T
must be in Relin
2 nRT
=
log [-]
Adiabatic M -
=
nCyΔT 2 0 =
Xu = -
W
a 0
=
Cy ER
=
(tre for any of these processes
You might also like
- ME 2131 Module PDFDocument64 pagesME 2131 Module PDFGreen Brain0% (1)
- Solution Manual for an Introduction to Equilibrium ThermodynamicsFrom EverandSolution Manual for an Introduction to Equilibrium ThermodynamicsNo ratings yet
- Gen2MR 31173Document12 pagesGen2MR 31173gtravaNo ratings yet
- Little Women PDFDocument684 pagesLittle Women PDFGeovanny MerinoNo ratings yet
- Robotics CH 4 Robot DynamicsDocument40 pagesRobotics CH 4 Robot DynamicsCharlette Alessi InaoNo ratings yet
- Thermodynamics: Mind MapsDocument1 pageThermodynamics: Mind MapsRiya BhardwajNo ratings yet
- DC Motors FormulasDocument3 pagesDC Motors FormulasSaabierah SalieNo ratings yet
- Capacitors & Capacitance 2024Document4 pagesCapacitors & Capacitance 2024royal01818No ratings yet
- Sodapdf-Merged 4Document5 pagesSodapdf-Merged 4harshrajclass9brollno.21No ratings yet
- Rumus FisikaDocument6 pagesRumus FisikaEvita CarolineNo ratings yet
- Thermodynamics: Work Done From P-V GraphDocument1 pageThermodynamics: Work Done From P-V Graphbhatr407No ratings yet
- 이름 없는 노트북Document3 pages이름 없는 노트북pilotcsh01No ratings yet
- Crash Course PDFDocument8 pagesCrash Course PDFSatvik SinghNo ratings yet
- Gas Power Cycles - 2Document6 pagesGas Power Cycles - 2Sehar FatimaNo ratings yet
- PhysicsDocument1 pagePhysicsAriel SantillanNo ratings yet
- PHYSchartDocument2 pagesPHYSchartSkander HamadiNo ratings yet
- Ramraj: SolutionDocument8 pagesRamraj: SolutionRajat Verma X D 39No ratings yet
- Electromagnetism Equation Sheet!!!!Document2 pagesElectromagnetism Equation Sheet!!!!Châu Lâm TuầnNo ratings yet
- Solutions & Colligative PropertiesDocument14 pagesSolutions & Colligative PropertiesPoonam PrasadNo ratings yet
- Fourier Series TableDocument3 pagesFourier Series TableAlshargabi MusaabNo ratings yet
- Hydrostatics ST2 SHC 310Document9 pagesHydrostatics ST2 SHC 310Kat DjordjevicNo ratings yet
- ProofsDocument6 pagesProofskudoshinichiedogawaconan123No ratings yet
- Formula Sheet EE-434 Power Electronics: Basic Expressions CapacitorDocument3 pagesFormula Sheet EE-434 Power Electronics: Basic Expressions CapacitorMuhammad Muzammil SaleemNo ratings yet
- 无标题的笔记本Document6 pages无标题的笔记本shuaicheng geNo ratings yet
- Ideal Gas ProcessesDocument6 pagesIdeal Gas ProcessesKlydeJoseNo ratings yet
- 02 09 22Document5 pages02 09 22Mahima AroraNo ratings yet
- TD Introduction To Quantum PhysicsDocument11 pagesTD Introduction To Quantum PhysicsusaroufNo ratings yet
- Sample Formula Sheet FinalDocument3 pagesSample Formula Sheet Finalanmol singhNo ratings yet
- Assignment 4Document19 pagesAssignment 4Radhe JhaNo ratings yet
- Physics Formula MidsemDocument4 pagesPhysics Formula MidsemWAN MUHAMMAD IRFAN WAN MOHD ZOHARNo ratings yet
- Thermodynamics Property Tables PDFDocument19 pagesThermodynamics Property Tables PDFHueHue HueNo ratings yet
- Mechanics of Materials Equation SheetDocument3 pagesMechanics of Materials Equation SheetAramis Kelkelyan100% (1)
- Physics Notes#1 Circular MotionDocument5 pagesPhysics Notes#1 Circular MotiongdsutaNo ratings yet
- Ln3.Fm# - Fcoulomb 4Tfeo&Z: BalmerDocument3 pagesLn3.Fm# - Fcoulomb 4Tfeo&Z: BalmerJayNo ratings yet
- Assignment 1Document13 pagesAssignment 1Jaco GreeffNo ratings yet
- Bahas SIMAK Fisika - 260623Document16 pagesBahas SIMAK Fisika - 260623gerry_liyanaNo ratings yet
- Flexibility Composite StudentDocument4 pagesFlexibility Composite StudentMuhammad IqbalNo ratings yet
- Biothermo Cheatsheet Copy DESKTOP BLE90C7Document5 pagesBiothermo Cheatsheet Copy DESKTOP BLE90C7Pay XinniNo ratings yet
- 電動力學 L1Document18 pages電動力學 L1盧郁傑No ratings yet
- Clase 11 Fisica ModernaDocument2 pagesClase 11 Fisica ModernanoelNo ratings yet
- Formula Lab Sheet - 1p22 PDFDocument2 pagesFormula Lab Sheet - 1p22 PDFRoy VeseyNo ratings yet
- Population: BinomialDocument10 pagesPopulation: Binomialnaghamaoun.13No ratings yet
- Germany: ConnectingDocument7 pagesGermany: ConnectingFranco Claudio Antonio Porras YarascaNo ratings yet
- Tema 8 FísicaDocument4 pagesTema 8 FísicaNaviroNo ratings yet
- 공기수1 - 박준현 - 정리노트Document19 pages공기수1 - 박준현 - 정리노트jonnabisaaNo ratings yet
- Legea Lui Maxwell Pentru Distribuția Moleculelor După Vitezele Lor RelativeDocument3 pagesLegea Lui Maxwell Pentru Distribuția Moleculelor După Vitezele Lor RelativecristianNo ratings yet
- Clase Del 12-02-2021Document3 pagesClase Del 12-02-2021EdwinNo ratings yet
- HW 1Document3 pagesHW 1kako3163No ratings yet
- Ec1 FormulaDocument10 pagesEc1 Formulaborja familyNo ratings yet
- Periodic Motion NotesDocument9 pagesPeriodic Motion Noteschlorine169No ratings yet
- 29 05 ElivDocument13 pages29 05 ElivTapan BadheiNo ratings yet
- Antenna Gain Isotropic 1.00 Half-Wave Dipole 1.64 Elementary Doublet 1.5Document2 pagesAntenna Gain Isotropic 1.00 Half-Wave Dipole 1.64 Elementary Doublet 1.5Iñaki Zuriel ConstantinoNo ratings yet
- Ejercicios de FísicaDocument25 pagesEjercicios de FísicaVictor MaidanaNo ratings yet
- Lumped CapacitanceDocument4 pagesLumped CapacitanceMustafe Sheikh MohamedNo ratings yet
- 351 F 22 Exam EquationsDocument1 page351 F 22 Exam EquationsEdaNo ratings yet
- TheoryDocument23 pagesTheorykzdvfq7vk4No ratings yet
- How To Select Overcurrent Relay CharacteristicsDocument11 pagesHow To Select Overcurrent Relay CharacteristicsRogelio RevettiNo ratings yet
- 491-Theory Exercise 1Document4 pages491-Theory Exercise 1Arundhati GuptaNo ratings yet
- Formular 1Document1 pageFormular 1พิมพ์ โพธิสัตว์No ratings yet
- Energy TransportDocument16 pagesEnergy TransportSarun NeamnomNo ratings yet
- The Spectral Theory of Toeplitz Operators. (AM-99), Volume 99From EverandThe Spectral Theory of Toeplitz Operators. (AM-99), Volume 99No ratings yet
- The Equidistribution Theory of Holomorphic Curves. (AM-64), Volume 64From EverandThe Equidistribution Theory of Holomorphic Curves. (AM-64), Volume 64No ratings yet
- Cardiovascular SystemDocument1 pageCardiovascular SystemYu YuNo ratings yet
- Brain ?Document5 pagesBrain ?Yu YuNo ratings yet
- Oral Route of AdministrationDocument1 pageOral Route of AdministrationYu YuNo ratings yet
- Katwalker11 - Nervous System IDocument4 pagesKatwalker11 - Nervous System IYu YuNo ratings yet
- Intravenous Route of AdministrationDocument2 pagesIntravenous Route of AdministrationYu YuNo ratings yet
- Rectal AdministrationDocument2 pagesRectal AdministrationYu YuNo ratings yet
- Present Simple and PastDocument4 pagesPresent Simple and PastYu YuNo ratings yet
- Plasmodium: Aye Chan Myat Phyo Nang Moe Pyae Pyae Hein Shwe Pwint Pyae Sone Yoon Su Nadi Yin Nyein HanDocument8 pagesPlasmodium: Aye Chan Myat Phyo Nang Moe Pyae Pyae Hein Shwe Pwint Pyae Sone Yoon Su Nadi Yin Nyein HanYu YuNo ratings yet
- 19Document1 page19Yu YuNo ratings yet
- Week 12 Myp 4 HWDocument2 pagesWeek 12 Myp 4 HWAnchal ChadhaNo ratings yet
- Pre Stressed Concrete Manual Computer Applications On SAP2000, ETABSDocument259 pagesPre Stressed Concrete Manual Computer Applications On SAP2000, ETABSGardener AyuNo ratings yet
- AR 303 Vol 1 DDocument968 pagesAR 303 Vol 1 DAnonymous gM6RZL5lYdNo ratings yet
- CHM 101 QuestionsDocument4 pagesCHM 101 QuestionsAbraham BanjoNo ratings yet
- Methods To Determine The Elastic Line: Description Learning Objectives/experimentsDocument3 pagesMethods To Determine The Elastic Line: Description Learning Objectives/experimentsDiego AvendañoNo ratings yet
- Xii Physics Experiment 1Document5 pagesXii Physics Experiment 1Solomon Peter SunilNo ratings yet
- Tutorial-Lateral Earth PressureDocument4 pagesTutorial-Lateral Earth PressuremanarajNo ratings yet
- SPM Phy Quantity of Heat IDocument13 pagesSPM Phy Quantity of Heat ICHINEMEREM EZEHNo ratings yet
- User Manual DPS - ENDocument60 pagesUser Manual DPS - ENYiannis Steletaris100% (1)
- Refrigeration Troubleshooting GuideDocument8 pagesRefrigeration Troubleshooting GuideJuan Jose Ramirez CorralesNo ratings yet
- Foundationxpart 1Document3 pagesFoundationxpart 1Haydeesheen SisonNo ratings yet
- HQ Series InsertDocument19 pagesHQ Series InsertAnderson ReisNo ratings yet
- MD Product Catalogue PROXIMITY ENGDocument194 pagesMD Product Catalogue PROXIMITY ENGETIENNENo ratings yet
- PowerLogic ION8650 - M8650A0C0H5E1B0ADocument4 pagesPowerLogic ION8650 - M8650A0C0H5E1B0AKevin GurungNo ratings yet
- Physical Science - Semester End Project: The Electromagnetic SpectrumDocument4 pagesPhysical Science - Semester End Project: The Electromagnetic SpectrumBreanna HartNo ratings yet
- Modulation Schemes of The Three-Phase Impedance Source Inverters - Part II: Comparative AssessmentDocument13 pagesModulation Schemes of The Three-Phase Impedance Source Inverters - Part II: Comparative Assessmentek9925No ratings yet
- HK MalikDocument840 pagesHK MalikHarsan Singh100% (1)
- Detailed Design Study of North Java Corridor Flyover Project Balaraja Flyover Detailed Design SubstructureDocument228 pagesDetailed Design Study of North Java Corridor Flyover Project Balaraja Flyover Detailed Design SubstructureNetzoo FlixNo ratings yet
- Covalent Bonding: Mrs. C. Williams-Massey Third From ChemistryDocument15 pagesCovalent Bonding: Mrs. C. Williams-Massey Third From ChemistryJoshua BrownNo ratings yet
- Vegetation Patterns Along A Rainfall Gradient: Ehud Meron, Erez Gilad, Jost Von Hardenberg, Moshe Shachak, Yair ZarmiDocument10 pagesVegetation Patterns Along A Rainfall Gradient: Ehud Meron, Erez Gilad, Jost Von Hardenberg, Moshe Shachak, Yair ZarmiEmmanuel kpegloNo ratings yet
- 0234 - Chimney Structural - R0Document7 pages0234 - Chimney Structural - R0Rufus D SNo ratings yet
- Pressure - Enthalpy Diagram For The Refrigerant R-22: Li Q U IdDocument1 pagePressure - Enthalpy Diagram For The Refrigerant R-22: Li Q U IdRifki AuliaNo ratings yet
- Achenbach - 1995 - Heat and Flow Characteristics of Packed BedsDocument11 pagesAchenbach - 1995 - Heat and Flow Characteristics of Packed BedsDOUGLAS RAMON RODRIGUEZ ORDOÑEZNo ratings yet
- Airflow Sensors AWM5000 Series High Flow Mass Airflow/AmplifiedDocument4 pagesAirflow Sensors AWM5000 Series High Flow Mass Airflow/AmplifiedwidsonmeloNo ratings yet
- Cardinal Points and Types of NorthDocument18 pagesCardinal Points and Types of NorthKrishnanshu MishraNo ratings yet
- DTR 1500 Eng - 12.2019Document39 pagesDTR 1500 Eng - 12.2019diogo_airjNo ratings yet
- International Journal of Biological MacromoleculesDocument10 pagesInternational Journal of Biological MacromoleculesAlejandra Gonzalez RuizNo ratings yet