Professional Documents
Culture Documents
Chemistry H.W.
Chemistry H.W.
Uploaded by
Tarouni .MOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chemistry H.W.
Chemistry H.W.
Uploaded by
Tarouni .MCopyright:
Available Formats
Q1/
1. Isotopes are atoms of the same element that have different numbers of neutrons,
but the same number of protons and electrons.
Example:
Tritium is an isotope of hydrogen and is used to make things such as clock faces
and wristwatches glow in the dark. Tritium provides an extremely bright self-
activated, self-sustaining light source that will stay bright throughout the night
and has a life span of twenty years.
2. also called atomic mass number or nucleon number, is the total number of protons
and neutrons (together known as nucleons) in an atomic nucleus.
Example:
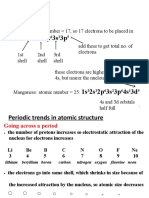
3717Cl has a mass number of 37. Its nucleus contains 17 protons and 20 neutrons.
The mass number of carbon-13 is 13. When a number is given following an element
name, this is its isotope, which basically states the mass number. To find the
number of neutrons in an atom of the isotope, simply subtract the number of protons
(atomic number). So, carbon-13 has 7 neutrons, because carbon has atomic number 6.
3. The atomic number of a chemical element is the number of protons in the nucleus
of an atom of the element. It is the charge number of the nucleus since neutrons
carry no net electrical charge.
Example:
The atomic number of hydrogen is 1; the atomic number of carbon is 6, and the
atomic number of silver is 47: any atom with 47 protons is an atom of silver.
Varying the number of neutrons in an element changes its isotopes while changing
the numbers of electrons makes it an ion.
4. Ionic bonding is a type of chemical bonding that involves the electrostatic
attraction between oppositely charged ions, or between two atoms with sharply
different electronegativities, and is the primary interaction occurring in ionic
compounds.
Example:
NaBr: sodium bromide
KBr: potassium bromide
NaCl: sodium chloride
NaF: sodium fluoride
5. The tendency of an atom in a molecule to attract the shared pair of electrons
towards itself is known as electronegativity.
Example:
The chlorine atom has a higher electronegativity than the hydrogen atom, so the
bonding electrons will be closer to the Cl than to the H in the HCl molecule. In
the O 2 molecule, both atoms have the same electronegativity. The electrons in the
covalent bond are shared equally between the two oxygen atoms.
6. Geometric isomers are chemical species with the same type and quantity of atoms
as one another, yet having different geometric structures. In geometric isomers,
atoms or groups exhibit different spatial arrangements on either side of a chemical
bond or ring structure.
Example:
Geometric Isomers cannot move freely due to rigid structures like carbon-carbon,
carbon-nitrogen, or nitrogen-nitrogen. Rigidity is due to a double bond. Some
geometrical isomerism examples are : stilbene, C14H12, a cyclic compound, rigid due
to the ring structure.
Q2/
1. Polar covalent bond
2. Ionic bonding
3. Covalent bond
4. Organic chemistry
5. Two ? bonds
6. Pi bond
7. Isomers
You might also like
- Bronchiolitis AAP 2021Document29 pagesBronchiolitis AAP 2021Natalie KwongNo ratings yet
- Chap 4 BIOLOGY BIODocument82 pagesChap 4 BIOLOGY BIOsarah575No ratings yet
- Properties of BondsDocument36 pagesProperties of BondsPaulNo ratings yet
- 14 Lewis Structures and Molecuar Models S19Document14 pages14 Lewis Structures and Molecuar Models S19victorNo ratings yet
- Chemical Bonding: Asst. Prof. Jean Theresa O. GoDocument51 pagesChemical Bonding: Asst. Prof. Jean Theresa O. GoOsannah Irish InsongNo ratings yet
- Review of Related Studies and LiteratureDocument5 pagesReview of Related Studies and LiteratureJoanalyn Lata100% (5)
- Chemical Bonding 1Document37 pagesChemical Bonding 1palal123No ratings yet
- RFF TB Units 1 6Document89 pagesRFF TB Units 1 6Lucia Silva0% (1)
- Unit 1 Molecules Their Interaction Relevant To Biology CSIR UGC NET Life SciencesDocument5 pagesUnit 1 Molecules Their Interaction Relevant To Biology CSIR UGC NET Life SciencesKishan KoyaniNo ratings yet
- Port Operations Manual 2002Document67 pagesPort Operations Manual 2002markie supieNo ratings yet
- Chapter 2 RevisedDocument39 pagesChapter 2 RevisedMohammed AllamNo ratings yet
- Aeration Tank Blower Calcns Package - US UnitsDocument19 pagesAeration Tank Blower Calcns Package - US UnitsAqua tech chemicals officesNo ratings yet
- Modules in Chemistry 2Document120 pagesModules in Chemistry 2Amanda WardNo ratings yet
- Chemistry in Focus A Molecular View of Our World 5Th Edition Tro Solutions Manual Full Chapter PDFDocument34 pagesChemistry in Focus A Molecular View of Our World 5Th Edition Tro Solutions Manual Full Chapter PDFjulianna.washington847100% (11)
- Protons, Neutrons & Electrons: Relative Atomic MassDocument19 pagesProtons, Neutrons & Electrons: Relative Atomic MassAiza ImranNo ratings yet
- Chemistry For EngineeringDocument3 pagesChemistry For EngineeringMarcRhyme CalaylayNo ratings yet
- Chemical Biology Lecture - REVIEWERDocument3 pagesChemical Biology Lecture - REVIEWERFranchiezca AoananNo ratings yet
- Bonding and Strucure: Igcse Co-Ordinated ScienceDocument87 pagesBonding and Strucure: Igcse Co-Ordinated ScienceNicholas ChenNo ratings yet
- Screenshot 2023-11-24 at 13.18.48Document69 pagesScreenshot 2023-11-24 at 13.18.48Lana MajidNo ratings yet
- Atomic StructureDocument11 pagesAtomic StructureEnergy KojoNo ratings yet
- Subatomic ParticlesDocument50 pagesSubatomic Particlesdreample1003No ratings yet
- Atomic Structure: Electron Proton NeutronDocument4 pagesAtomic Structure: Electron Proton NeutronTalao, Angelie Rei S.No ratings yet
- CHEM 205 Chapter 8 Section 12.3Document30 pagesCHEM 205 Chapter 8 Section 12.3phikjaeNo ratings yet
- Atomic Structure PDFDocument9 pagesAtomic Structure PDFPoojal BatraNo ratings yet
- Mariano Marcos State University: PCHM 121: Pharmaceutical Inorganic Chemistry With Qualitative AnalysisDocument14 pagesMariano Marcos State University: PCHM 121: Pharmaceutical Inorganic Chemistry With Qualitative AnalysisKaizenNo ratings yet
- Chemical Bond and Hybridization1Document27 pagesChemical Bond and Hybridization1diyarberwari15No ratings yet
- Class04 ChemistryG12 Notes and HomeworkDocument58 pagesClass04 ChemistryG12 Notes and HomeworkAndy Rei KouNo ratings yet
- Chapter 2Document48 pagesChapter 2lelouchali1234No ratings yet
- Questions 1Document25 pagesQuestions 1Sarupya TigutiNo ratings yet
- General IntroductionDocument6 pagesGeneral IntroductionTolani AyoNo ratings yet
- Chemical Bonding 2016Document75 pagesChemical Bonding 2016MBOTAKE LawsonNo ratings yet
- NEPHAR 109 - Chapter 2Document73 pagesNEPHAR 109 - Chapter 2Amirabbas SaffariNo ratings yet
- Chapter 1Document62 pagesChapter 1mortemsondeathNo ratings yet
- Chapter 2 - Introductory Chemistry & Biochemistry: Phosphate Head (Polar)Document12 pagesChapter 2 - Introductory Chemistry & Biochemistry: Phosphate Head (Polar)Rajarathinam1235463No ratings yet
- Nonpolar Covalent Bonds: ChemistryDocument9 pagesNonpolar Covalent Bonds: ChemistryRathnakrajaNo ratings yet
- Nonpolar Covalent Bonds: ChemistryDocument9 pagesNonpolar Covalent Bonds: ChemistryRathnakrajaNo ratings yet
- ° Polar and Non-Polar Covalent Bonds 3Document23 pages° Polar and Non-Polar Covalent Bonds 3Zayyan AliNo ratings yet
- Ch01-Introduction of Organic ChemistryDocument47 pagesCh01-Introduction of Organic ChemistrySİNEM GÜVENNo ratings yet
- Atomic Structure and Chemical BondingDocument4 pagesAtomic Structure and Chemical Bondinganshvishwakarma674100% (1)
- Campbell Lecture Notes Chemistry of LifeDocument42 pagesCampbell Lecture Notes Chemistry of LifeSophia Andrei VillalunaNo ratings yet
- ATOMic StructureDocument5 pagesATOMic Structuretalithaonkabetse723No ratings yet
- Chemical Bonds: Frederick A. Bettelheim William H. Brown Mary K. Campbell Shawn O. Farrell Omar J. TorresDocument48 pagesChemical Bonds: Frederick A. Bettelheim William H. Brown Mary K. Campbell Shawn O. Farrell Omar J. TorresBERNA MAE TAMAYONo ratings yet
- Molecular StructureDocument31 pagesMolecular Structurefitria faizNo ratings yet
- Biology 2E: Chapter 2 The Chemical Foundation of LifeDocument31 pagesBiology 2E: Chapter 2 The Chemical Foundation of LifeTaya LewendonNo ratings yet
- Chemistry - Carbon and Its Compounds - Class Notes - WARRIOR SERIES CLASS-10THDocument136 pagesChemistry - Carbon and Its Compounds - Class Notes - WARRIOR SERIES CLASS-10THashudavid126474No ratings yet
- Da-Voc-Abe 3.1.1Document25 pagesDa-Voc-Abe 3.1.1godfrey rufusNo ratings yet
- Chemical Bonding Premier NotesDocument55 pagesChemical Bonding Premier NotesGaurab BhattaraiNo ratings yet
- CDBCEB1013 Lecture 1 PDFDocument87 pagesCDBCEB1013 Lecture 1 PDFPutri Nur Aisyah Halmy AzamNo ratings yet
- Mendeleev's Periodic Table of The ElementsDocument20 pagesMendeleev's Periodic Table of The ElementsMohammed TarekNo ratings yet
- Bonding in Organic Compounds: Chapter SummaryDocument390 pagesBonding in Organic Compounds: Chapter SummaryGlyzen GaleonNo ratings yet
- Structure of Substance - Lesson - 2Document10 pagesStructure of Substance - Lesson - 2samsonNo ratings yet
- Bai GiangDocument104 pagesBai GiangminhyNo ratings yet
- 1s 2s 2p 3s 3p: ExamplesDocument104 pages1s 2s 2p 3s 3p: ExamplesminhyNo ratings yet
- Atomic Structure and Chemical Bonding Exercise Ex. 4 (C)Document8 pagesAtomic Structure and Chemical Bonding Exercise Ex. 4 (C)Harshita ChoudharyNo ratings yet
- Chemical Bonding Chemistry 1C Engr. Albert S. Revilla InstructorDocument3 pagesChemical Bonding Chemistry 1C Engr. Albert S. Revilla Instructorgeng gengNo ratings yet
- Chemistry 9th Edition Zumdahl Solutions ManualDocument35 pagesChemistry 9th Edition Zumdahl Solutions Manualstrewmerils1ej3n100% (14)
- Dwnload Full Chemistry 9th Edition Zumdahl Solutions Manual PDFDocument35 pagesDwnload Full Chemistry 9th Edition Zumdahl Solutions Manual PDFelijah3oa4knight100% (13)
- General ChemistryDocument6 pagesGeneral ChemistryJewel ValenciaNo ratings yet
- 4 Chemical Bonding - After Review - 8!10!2019Document24 pages4 Chemical Bonding - After Review - 8!10!2019AFAQ HYDNo ratings yet
- Study Materials: Vedantu Innovations Pvt. Ltd. Score High With A Personal Teacher, Learn LIVE Online!Document26 pagesStudy Materials: Vedantu Innovations Pvt. Ltd. Score High With A Personal Teacher, Learn LIVE Online!Bhupinder KaurNo ratings yet
- Chemistry of LifeDocument14 pagesChemistry of LifeBayramNo ratings yet
- Chemistry of LifeDocument14 pagesChemistry of LifeRebecca TercianoNo ratings yet
- TEX - CHEM 103 Organic ChemistryDocument52 pagesTEX - CHEM 103 Organic ChemistrychioNo ratings yet
- Lecture 2 Atomic StructureDocument32 pagesLecture 2 Atomic StructureAhmed FouadNo ratings yet
- People of The Philippines v. OritaDocument6 pagesPeople of The Philippines v. OritaJoyce VillanuevaNo ratings yet
- 2 Girth Gear Asset ManagementDocument32 pages2 Girth Gear Asset ManagementAnesu ChimhowaNo ratings yet
- Daihan Premium Pro-Microcentrifuge Set ,: Max. 13,500 RPMDocument1 pageDaihan Premium Pro-Microcentrifuge Set ,: Max. 13,500 RPMRedmi TigaesNo ratings yet
- Dr. Eman El Sawaf: Anatomy & Embryology DepartmentDocument93 pagesDr. Eman El Sawaf: Anatomy & Embryology DepartmentKareem DawoodNo ratings yet
- Two Wheeler Insurance Package Policy (1) - Insurance - ChequeDocument5 pagesTwo Wheeler Insurance Package Policy (1) - Insurance - ChequepriyanshNo ratings yet
- Royal Dutch Shell PLC Investor's Handbook 2010-2014: Consolidated Balance Sheet (At December 31)Document1 pageRoyal Dutch Shell PLC Investor's Handbook 2010-2014: Consolidated Balance Sheet (At December 31)Shara ValleserNo ratings yet
- UntitledDocument468 pagesUntitledIndesign IndeNo ratings yet
- LH800 Geogrid For Earth Reinforcement: The Engineered AdvantageDocument2 pagesLH800 Geogrid For Earth Reinforcement: The Engineered AdvantagetodoransNo ratings yet
- STEP 1 - SITE INSPECTION (Risk Assessment Form - Appendix A1)Document4 pagesSTEP 1 - SITE INSPECTION (Risk Assessment Form - Appendix A1)AlbertoAkpoghenetaNo ratings yet
- POFA Practice QuestionDocument36 pagesPOFA Practice QuestionAli Zain ParharNo ratings yet
- I 3974 Audio Mixer ManualDocument40 pagesI 3974 Audio Mixer ManualAnonymous aP1FSUPoNo ratings yet
- Adolescent Romantic Love A PHDocument198 pagesAdolescent Romantic Love A PHmiclescu100% (1)
- Ayurvedic Diagnostic & Management Approach-FinalDocument15 pagesAyurvedic Diagnostic & Management Approach-FinalMahesh TaleNo ratings yet
- Resistoflex Rubber PadsDocument2 pagesResistoflex Rubber PadsAnderson SNo ratings yet
- Meal Plan Form (Sanchez, Joshua V.)Document2 pagesMeal Plan Form (Sanchez, Joshua V.)Joshua Valencia SanchezNo ratings yet
- Isbell Young 1996Document9 pagesIsbell Young 1996johnny cartinNo ratings yet
- KG 98-2-ONT-ME-OGT-DS-00001 - Data Sheet For Gas Separator (V-N78152 AB) Rev.0Document7 pagesKG 98-2-ONT-ME-OGT-DS-00001 - Data Sheet For Gas Separator (V-N78152 AB) Rev.0sumit kumarNo ratings yet
- Taverna - Georgina HaydenDocument403 pagesTaverna - Georgina Haydenelena8kartsiouliNo ratings yet
- Arrival Registration A27395003Document1 pageArrival Registration A27395003Watch ShamsNo ratings yet
- OOS - 2021 - 22 - UAE SST TERM 3 - LESSON 2 - 21st Cetury Infrastructure and Smart Initiatives-AnswerkeyDocument3 pagesOOS - 2021 - 22 - UAE SST TERM 3 - LESSON 2 - 21st Cetury Infrastructure and Smart Initiatives-AnswerkeyLiterally just ReysNo ratings yet
- BOSH - Lecture 9 - Personal Protective EquipmentDocument11 pagesBOSH - Lecture 9 - Personal Protective EquipmentAlfonso Martin AngelesNo ratings yet
- 4 - Kiehn, B & Swales, M. (2007) - An Overview of Dialectical Behaviour Therapy in The Treatment ofDocument9 pages4 - Kiehn, B & Swales, M. (2007) - An Overview of Dialectical Behaviour Therapy in The Treatment ofAnastasiya PlohihNo ratings yet
- Running Head: Contagion Movie Assessment 1Document5 pagesRunning Head: Contagion Movie Assessment 1swathim123No ratings yet
- ACLEIADocument374 pagesACLEIAshariq begNo ratings yet
- Nit KRDocument56 pagesNit KRindians jonesNo ratings yet