Professional Documents
Culture Documents
Equilibrium: Is by in Pi
Equilibrium: Is by in Pi
Uploaded by
HANNAH ZAFIRAH MUHAMAD ZAIDIOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Equilibrium: Is by in Pi
Equilibrium: Is by in Pi
Uploaded by
HANNAH ZAFIRAH MUHAMAD ZAIDICopyright:
Available Formats
CHAPTER 5 PHASE EQUILIBRIUM
Degree of freedom f f z p
p noof phase
phase Diagram veslope forward lefttoright M
veslope backward righttoleft TP
external MP
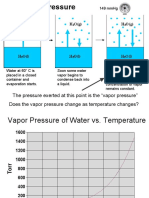
Raoult's law states that partialvapour pressure ofcomponent air in a solution at a giventemperature is equal to the
molefraction ofAlbinthesolution multiplied by thevapour pressure of pure air atthesametemperature
in upon
miscible ideal liquidsA B total pressure of thevapourPi abovetheliquid mixture is thesum of t
partial pressure ofthetwo vapoursPa PB
Dalton's
law totalvapour pressure is equal tothe sum ofthepartial vapour pressure at that composition
p P tPB t
vapour composition vapour rich in more volatile component
moretraction
it s s moles of A
Y totalnoofmoles Atb
fractional Distillation procedure
for separating mixtures ofvolatile components based on difference in BP
Principlea Distillate lower Br Downlower
ciiiresidue nigher BP rise Higher
condensethevapour andreboil it new vapour is evenricher in water
conigative properties
inlowering of vapour pressure P Ciii Depression of freezingpoint is
P spon pa is if if
Psointion
Xsolvent solvent
ii elevation ofboiling point in civosmoticpressure
in sibesolution iisolvent sMrii
in sima
im moiofsolute
imma
massofsolvent
You might also like
- QuizBowl QuestionsDocument76 pagesQuizBowl Questionsedmark icalina50% (4)
- Chapter 1 DistillationDocument73 pagesChapter 1 DistillationNUR HIDAYAHNo ratings yet
- Solution: STR Ycl AssesDocument40 pagesSolution: STR Ycl AssesArka DeyNo ratings yet
- Raoults LawDocument12 pagesRaoults LawZaqiya zahwa alifaNo ratings yet
- Chemistry Q2Document3 pagesChemistry Q2Kristel Anne Magaru-Macauggal-LugoNo ratings yet
- Simple Mixtures Colligative Properties: Chapter 7: SlideDocument32 pagesSimple Mixtures Colligative Properties: Chapter 7: SlideputriNo ratings yet
- Che 2Document25 pagesChe 2Jaynie Lee VillaranNo ratings yet
- Chapter 13-L2 - Ions in Aqueous Solutions and Colligative PropertiesDocument11 pagesChapter 13-L2 - Ions in Aqueous Solutions and Colligative PropertiesMaryam Mohammad Al AlamiNo ratings yet
- Raoult's Law 1Document12 pagesRaoult's Law 1Chelsea SeepersadNo ratings yet
- ביופיזיקהDocument10 pagesביופיזיקהsharonzechariaNo ratings yet
- Chapter 3, Unit 3, Pharmaceutical Analysis, B Pharmacy 1st Sem, Carewell PharmaDocument7 pagesChapter 3, Unit 3, Pharmaceutical Analysis, B Pharmacy 1st Sem, Carewell Pharmavenkat ramanaNo ratings yet
- Design Calculations: Optimization 3: Material Balance CalculationDocument15 pagesDesign Calculations: Optimization 3: Material Balance CalculationMvelo PhungulaNo ratings yet
- Properties of MixtureDocument26 pagesProperties of MixtureDuy Anh ĐàoNo ratings yet
- AC Lab 4 Molecular Weight Freezing Point I SDocument12 pagesAC Lab 4 Molecular Weight Freezing Point I SSSSSE7ENNo ratings yet
- Chem Eqm and Reaction Kinetics TopicalDocument12 pagesChem Eqm and Reaction Kinetics TopicalAaditya MKNo ratings yet
- Colligative PropertyDocument26 pagesColligative PropertyKshama SharmaNo ratings yet
- Meeting 6-7Document30 pagesMeeting 6-7Aldo FirmansyahNo ratings yet
- CHP 8 RejectDocument11 pagesCHP 8 RejectmuraliMuNo ratings yet
- CN3132 NotesDocument17 pagesCN3132 NotesShaunny BravoNo ratings yet
- Random Notes Class 12 ChemistryDocument87 pagesRandom Notes Class 12 Chemistryankitajamatia06No ratings yet
- 3 Hydrocarbon Phase BehaviourDocument45 pages3 Hydrocarbon Phase BehaviourMD. ASIF ALL AZADNo ratings yet
- QuizBowl QuestionsDocument84 pagesQuizBowl QuestionsJowel MercadoNo ratings yet
- Instrumentation and Control of Distillation TowersDocument20 pagesInstrumentation and Control of Distillation TowersHenry OkoyeNo ratings yet
- Solutions Notes PDFDocument21 pagesSolutions Notes PDFSMELLY CATNo ratings yet
- ChE ReviewerDocument82 pagesChE ReviewerKristineNo ratings yet
- Chem ENgg Board Exam QuestionsDocument42 pagesChem ENgg Board Exam QuestionsAllyana Marie TiemsimNo ratings yet
- Solutions: Roult's LawDocument2 pagesSolutions: Roult's LawSsNo ratings yet
- Brief Summary Sheet - AP ChemDocument5 pagesBrief Summary Sheet - AP ChemracheljchongNo ratings yet
- Colligative Properties: Vapour Pressure Boiling Point Freezing Point Osmotic PressureDocument27 pagesColligative Properties: Vapour Pressure Boiling Point Freezing Point Osmotic PressureRSNo ratings yet
- Chemical Engineering and Physical and Chemical Principles QuestionsDocument84 pagesChemical Engineering and Physical and Chemical Principles QuestionsMark Vincent EspinosaNo ratings yet
- Chemistry: SolutionDocument68 pagesChemistry: SolutionSatyajit RoutNo ratings yet
- Raoult's LawDocument8 pagesRaoult's LawHARSH DHOLAKIYANo ratings yet
- Thermo II Lecture 3 VaporLiquidEuilibria PDFDocument39 pagesThermo II Lecture 3 VaporLiquidEuilibria PDFJerome JavierNo ratings yet
- Solutions - Short Notes - Lakshya JEE 2024Document2 pagesSolutions - Short Notes - Lakshya JEE 2024suzalaggarwalllNo ratings yet
- Unit 9 Lecture Day 4-Colligative PropertiesDocument41 pagesUnit 9 Lecture Day 4-Colligative PropertiesPutri Nur AuliyaNo ratings yet
- Solution in One PageDocument2 pagesSolution in One Pageraiprisha06No ratings yet
- Estrazione Liq Liq 2Document16 pagesEstrazione Liq Liq 2x비No ratings yet
- SMB 2 Xii Chem Mod3Document20 pagesSMB 2 Xii Chem Mod3vijayaraipellyNo ratings yet
- Solubility Lect 4 & 5Document50 pagesSolubility Lect 4 & 5ketantchaudhariNo ratings yet
- 10 AzeotropeDocument24 pages10 AzeotropeXclipsionNo ratings yet
- Vapor-Liquid Equilibria: Introduction To Chemical Engineering CalculationsDocument47 pagesVapor-Liquid Equilibria: Introduction To Chemical Engineering CalculationsViet NguyenNo ratings yet
- 2001 SolutionDocument12 pages2001 SolutionSaghar FaridNo ratings yet
- Mod 3.2 Colligative, Tonicity and Mod 4 Solubility (PDF - Io)Document62 pagesMod 3.2 Colligative, Tonicity and Mod 4 Solubility (PDF - Io)Charles DapitoNo ratings yet
- Hukum RoultDocument25 pagesHukum RoultZaqiya zahwa alifaNo ratings yet
- Solution Part 4Document92 pagesSolution Part 4abuthahir1.mcaNo ratings yet
- Colligative Properties: Vapour Pressure Boiling Point Freezing Point Osmotic PressureDocument27 pagesColligative Properties: Vapour Pressure Boiling Point Freezing Point Osmotic PressureNaman GuptaNo ratings yet
- Volumetric Properties of Pure FluidsDocument38 pagesVolumetric Properties of Pure FluidsViren ParmarNo ratings yet
- Microbiology Serology PraticalDocument7 pagesMicrobiology Serology PraticalSiva RajanNo ratings yet
- Liquid Solution (13th)Document19 pagesLiquid Solution (13th)Raju SinghNo ratings yet
- Methods of PuriDocument10 pagesMethods of PuriZirwaNo ratings yet
- Liquid SolutionDocument16 pagesLiquid SolutionRaju SinghNo ratings yet
- Theory of Dilute Solution: Colligative PropertiesDocument33 pagesTheory of Dilute Solution: Colligative PropertiesRONTU SARKERNo ratings yet
- 12 Chemistry Keypoints SolutionDocument3 pages12 Chemistry Keypoints Solutionrubysenthil4No ratings yet
- Raoult's Law123Document25 pagesRaoult's Law123Abdur RehmanNo ratings yet
- CHE CHM QuestionsDocument84 pagesCHE CHM QuestionsCamille LeiNo ratings yet
- Plant PhysiologyDocument10 pagesPlant PhysiologyShalini VermaNo ratings yet
- G19RA Chap4 - Phase BehaviourDocument46 pagesG19RA Chap4 - Phase BehaviourReda Abdel AzimNo ratings yet