Professional Documents
Culture Documents
Thermodynamics
Uploaded by
Daniyal FaisalOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Thermodynamics
Uploaded by
Daniyal FaisalCopyright:
Available Formats
THERMODYNAMICS
2 (a) The first law of thermodynamics may be expressed as
U = (+q) + (+w)
where U is the increase in internal energy of the system.
State the meaning of:
+q ..............................................................................................................................................
...................................................................................................................................................
+w. ............................................................................................................................................
...................................................................................................................................................
[2]
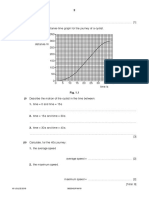
(b) The variation with pressure p of the volume V of a fixed mass of an ideal gas is shown in
Fig. 2.1.
4.0
B
V / 10 –3 m3
3.6
3.2
2.8
2.4
A C
2.0
2.2 2.6 3.0 3.4 3.8 4.2 4.6 5.0 5.4
p / 105 Pa
Fig. 2.1
The gas undergoes a cycle of changes A to B to C to A.
During the change A to B, the volume of the gas increases from 2.3 × 10–3 m3 to 3.8 × 10–3 m3.
© UCLES 2020 9702/41/O/N/20
7
(i) Show that the magnitude of the work done during the change A to B is 390 J.
[1]
(ii) State and explain the total change, if any, in the internal energy of the gas during one
complete cycle.
...........................................................................................................................................
...........................................................................................................................................
..................................................................................................................................... [2]
(c)
During the change B to C, no thermal energy enters or leaves the gas. The work done on the
gas during this change is 550 J.
Use these data and the information in (b) to complete Table 2.1.
Table 2.1
change q/J w/J U/J
A to B
.......................... .......................... ..........................
B to C
.......................... .......................... ..........................
C to A
.......................... .......................... ..........................
[4]
[Total: 9]
© UCLES 2020 9702/41/O/N/20 [Turn over
You might also like
- Finite Element Analysis of Aircraft WingDocument17 pagesFinite Element Analysis of Aircraft WingSai Rahul100% (1)
- ICSE Class 10 Physics Question Paper Solution 2020Document27 pagesICSE Class 10 Physics Question Paper Solution 2020Harshu KNo ratings yet
- Retaining Wall Design Based On ACI 318-14: Input Data & Design SummaryDocument3 pagesRetaining Wall Design Based On ACI 318-14: Input Data & Design SummaryAshishNo ratings yet
- IDT FoundationDocument15 pagesIDT FoundationARUN RAWATNo ratings yet
- Physics-0625-Paper-4 2023Document16 pagesPhysics-0625-Paper-4 2023Kasumi Sato60% (5)
- Physics Higher Level Paper 2: Instructions To CandidatesDocument24 pagesPhysics Higher Level Paper 2: Instructions To CandidatesdhanyaNo ratings yet
- Pelevic LNG Flow Module v2Document40 pagesPelevic LNG Flow Module v2DiwanEfendiEfendiNo ratings yet
- Cambridge IGCSE: CHEMISTRY 0620/42Document13 pagesCambridge IGCSE: CHEMISTRY 0620/42Rodolph Smith67% (3)
- Physics Standard Level Paper 2: Instructions To CandidatesDocument20 pagesPhysics Standard Level Paper 2: Instructions To CandidatesKevin HuNo ratings yet
- Set 62 - Chapter 2Document12 pagesSet 62 - Chapter 2lelon81No ratings yet
- Paper2 1999Document2 pagesPaper2 1999vasudhaNo ratings yet
- Cambridge International General Certifi Cate of Secondary EducationDocument12 pagesCambridge International General Certifi Cate of Secondary EducationDark GreenNo ratings yet
- Chemistry HL P2Document12 pagesChemistry HL P2Juan Fernando Velasco ForeroNo ratings yet
- 0620 m15 QP 32Document12 pages0620 m15 QP 32Jean LeeNo ratings yet
- Cambridge O Level: PHYSICS 5054/22Document16 pagesCambridge O Level: PHYSICS 5054/22Ved P4h PratapNo ratings yet
- Cambridge International AS & A Level: CHEMISTRY 9701/23Document12 pagesCambridge International AS & A Level: CHEMISTRY 9701/23johnNo ratings yet
- Cambridge International AS & A Level: CHEMISTRY 9701/23Document12 pagesCambridge International AS & A Level: CHEMISTRY 9701/23orisunayo olugbengaNo ratings yet
- Thermal Physics Assignment 2013Document10 pagesThermal Physics Assignment 2013asdsadNo ratings yet
- Thermodynamics WsDocument5 pagesThermodynamics Wsnturnaoglu25No ratings yet
- Thermodynamics 2Document14 pagesThermodynamics 2Alexandros KouretsisNo ratings yet
- 2.5 Evaporation and Boiling Set 3 QP MsDocument10 pages2.5 Evaporation and Boiling Set 3 QP Msbipin jainNo ratings yet
- 9701/23/M/J/20 © Ucles 2020Document10 pages9701/23/M/J/20 © Ucles 2020Fire stormNo ratings yet
- 2021 Sda F5 June Test 1Document11 pages2021 Sda F5 June Test 1blueegofxNo ratings yet
- EquilibriumDocument15 pagesEquilibriumHala NajiNo ratings yet
- Cambridge IGCSE: PHYSICS 0625/43Document16 pagesCambridge IGCSE: PHYSICS 0625/43Krisha ElangovanNo ratings yet
- Screenshot 2023-10-03 at 9.41.01 PMDocument100 pagesScreenshot 2023-10-03 at 9.41.01 PM8f7cbjgh6vNo ratings yet
- Topic 3 Test - Paper 2Document2 pagesTopic 3 Test - Paper 2IB BaddiesNo ratings yet
- 4 Ideal Gas0001Document18 pages4 Ideal Gas0001Pooja MehraNo ratings yet
- CH 8 3Document9 pagesCH 8 3Muhammad ZainNo ratings yet
- 9701 - s17 - QP - 42 CharlesDocument30 pages9701 - s17 - QP - 42 Charlescharlesma123No ratings yet
- 9701 s17 QP 42 RemovedDocument18 pages9701 s17 QP 42 RemovedSherise EeNo ratings yet
- November 2020 Question Paper 11Document20 pagesNovember 2020 Question Paper 11a humanNo ratings yet
- ChemistryDocument4 pagesChemistryTamerlan KudaibergenNo ratings yet
- Assessment 1 Mechanics Part 1Document4 pagesAssessment 1 Mechanics Part 1Nirmani RodrigoNo ratings yet
- Safari PDFDocument13 pagesSafari PDFHussain KhanNo ratings yet
- Water 1Document10 pagesWater 12025svyasNo ratings yet
- Chem Ib PaperDocument18 pagesChem Ib Paperembededodin0% (1)
- Cambridge IGCSE: Environmental Management 0680/11Document20 pagesCambridge IGCSE: Environmental Management 0680/11rumaizaNo ratings yet
- May 2010 TZ2 Paper 2 PDFDocument10 pagesMay 2010 TZ2 Paper 2 PDFleanne yangNo ratings yet
- Cambridge O Level: PHYSICS 5054/02Document20 pagesCambridge O Level: PHYSICS 5054/02Arslan SarwarNo ratings yet
- A5. This Question Is About Energy Transfers.: Dynamo (Generator) Boiler CoalDocument1 pageA5. This Question Is About Energy Transfers.: Dynamo (Generator) Boiler CoalJorge ChavezNo ratings yet
- Cambridge IGCSE: CHEMISTRY 0620/42Document16 pagesCambridge IGCSE: CHEMISTRY 0620/42Renesh PatelNo ratings yet
- Chemistry SL P2Document7 pagesChemistry SL P2Juan Fernando Velasco ForeroNo ratings yet
- Cambridge O Level: Biology 5090/21Document16 pagesCambridge O Level: Biology 5090/21Rukmini BissoonauthNo ratings yet
- Thermal ExpansionDocument3 pagesThermal ExpansionAhmad Shafiq ZiaNo ratings yet
- Physics Paper TwoDocument11 pagesPhysics Paper Twohmatara8No ratings yet
- Energy Changesmj2020Document3 pagesEnergy Changesmj2020FenNo ratings yet
- 5090 s22 QP 22Document20 pages5090 s22 QP 22Tasnim Tabassum Promee 2031150649No ratings yet
- 16N Chemistry Paper 2 SLDocument16 pages16N Chemistry Paper 2 SLsvr5swxdjkNo ratings yet
- t2 Chem Revision Ex 12Document16 pagest2 Chem Revision Ex 12Nicholas OwNo ratings yet
- Cambridge O Level: Bangladesh Studies 7094/02Document28 pagesCambridge O Level: Bangladesh Studies 7094/02Prodoot BaruaNo ratings yet
- TransformationDocument11 pagesTransformationMuhammad DanishNo ratings yet
- My TestDocument8 pagesMy TestMarin PesicNo ratings yet
- DY Enthalpy TestDocument19 pagesDY Enthalpy TestStormzy 67No ratings yet
- Respiration Worksheet 2 With AnswersDocument12 pagesRespiration Worksheet 2 With AnswersshannonNo ratings yet
- Equilibria AsDocument39 pagesEquilibria AsyousafNo ratings yet
- Kami Export - Student-IsK Maryna Haponyuk 9D - Simple Kinetic Molecular Model of Matter 1 QPDocument9 pagesKami Export - Student-IsK Maryna Haponyuk 9D - Simple Kinetic Molecular Model of Matter 1 QPmarynashowNo ratings yet
- Review Pack 1st Term (23-24)Document21 pagesReview Pack 1st Term (23-24)Teck TieNo ratings yet
- Chemistry Paper 3 TZ1 SLDocument28 pagesChemistry Paper 3 TZ1 SLMotiani VanshikaNo ratings yet
- A-Level Ap1 Paper 1Document21 pagesA-Level Ap1 Paper 1Fasih AhmadNo ratings yet
- FORM 3 Phy PAPER 4Document22 pagesFORM 3 Phy PAPER 4Sabbath the sign of GodNo ratings yet
- 9702 Work Energy Power All Completed Upto May June 2011Document16 pages9702 Work Energy Power All Completed Upto May June 2011Sheeba HenryNo ratings yet
- Transition ElementsDocument21 pagesTransition ElementsextramemoryfordocsNo ratings yet
- Pages From 0625 - w15 - QP - 33-05Document1 pagePages From 0625 - w15 - QP - 33-05lelon ongNo ratings yet
- Student Exploration: Air TrackDocument8 pagesStudent Exploration: Air TrackTj alokolaro100% (2)
- CH2043 6 - Convective Heat Transfer Part 3 UpdatedDocument26 pagesCH2043 6 - Convective Heat Transfer Part 3 UpdatedNgọc Hà NguyễnNo ratings yet
- Performance Based Analysis of RC Struc With and Without Considering SsiDocument7 pagesPerformance Based Analysis of RC Struc With and Without Considering Ssijuan carlos molano toroNo ratings yet
- Flame PDFDocument2 pagesFlame PDFAlec joshua RapadaNo ratings yet
- Mechanics UNSEENDocument32 pagesMechanics UNSEENDulvan VitharanaNo ratings yet
- (Thesis) New Materials in Structural DesignDocument57 pages(Thesis) New Materials in Structural DesignShaileshRastogiNo ratings yet
- RC Desktop Toolkit v2Document14 pagesRC Desktop Toolkit v2Be Seang SeNo ratings yet
- CFD Assignment 2D Incompressible FlowDocument25 pagesCFD Assignment 2D Incompressible FlowPraphulNo ratings yet
- Balloon in A Bottle PDFDocument13 pagesBalloon in A Bottle PDFhusainiNo ratings yet
- Introduction To Planar Laser-Induced FlourescenceDocument3 pagesIntroduction To Planar Laser-Induced FlourescencegaccioNo ratings yet
- Protophobic Fifth Force Interpretation of The Observed Anomaly in Be Nuclear TransitionsDocument6 pagesProtophobic Fifth Force Interpretation of The Observed Anomaly in Be Nuclear Transitionsignacio rodriguezNo ratings yet
- GB 50135-2019 Standard For Design of High-Rising StructuresDocument164 pagesGB 50135-2019 Standard For Design of High-Rising StructuresAlec LiNo ratings yet
- C. W. PARK+G. M. HOMSY (1984) Two Phase Displacement in Hele Shaw Cells TheoryDocument18 pagesC. W. PARK+G. M. HOMSY (1984) Two Phase Displacement in Hele Shaw Cells TheoryLy HourNo ratings yet
- Lista ST Toys 52 1 Julio Descuento Hasta 60%Document583 pagesLista ST Toys 52 1 Julio Descuento Hasta 60%Mutual El CondorNo ratings yet
- A Broadband Low-Reflection Metamaterial AbsorberDocument6 pagesA Broadband Low-Reflection Metamaterial AbsorberRiston SinagaNo ratings yet
- FrictionDocument25 pagesFrictionRuby Rose de GuzmanNo ratings yet
- Assignment For Mid 1Document9 pagesAssignment For Mid 1Vũ Thị Hà TrangNo ratings yet
- Topic 4 Differential Pressure and Flow Sensors (Bernoulli Principle Venturi Effect)Document8 pagesTopic 4 Differential Pressure and Flow Sensors (Bernoulli Principle Venturi Effect)Vefa MustafazadeNo ratings yet
- H05514555 PDFDocument11 pagesH05514555 PDFPrakash InturiNo ratings yet
- Fluid Mechanics - LPP-1Document6 pagesFluid Mechanics - LPP-1Lester LewisNo ratings yet
- Physic Eng Mech StremaDocument9 pagesPhysic Eng Mech StremaAllyssa ErcillaNo ratings yet
- Steam TurbineDocument34 pagesSteam TurbineKRISHNA KANT GUPTANo ratings yet
- Mech-Intro 13.0 AppA BucklingDocument34 pagesMech-Intro 13.0 AppA Bucklingstathiss11No ratings yet
- St. Peter's College Sabayle ST., Poblacion, Iligan City: ObjectiveDocument3 pagesSt. Peter's College Sabayle ST., Poblacion, Iligan City: ObjectiveDonnald YambaNo ratings yet
- 01 Vibrations Lecture 1Document51 pages01 Vibrations Lecture 1Cody WaltonNo ratings yet