Professional Documents
Culture Documents
Spectroscopic Techniques For Pharmaceutical and Biopharmaceutical Industries - Unit 13 - Week 12
Uploaded by
antony bevanOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Spectroscopic Techniques For Pharmaceutical and Biopharmaceutical Industries - Unit 13 - Week 12
Uploaded by
antony bevanCopyright:
Available Formats
antonymichealbevan04@gmail.
com
NPTEL » Spectroscopic Techniques for
Pharmaceutical and Biopharmaceutical
Industries
Course outline
How does an NPTEL online course work?
Week 1
Week 2
Week 3
Week 4
Week 5
Week 6
Week 7
Week 8
Week 9
Week 10
Week 11
Week 12
Mass Spectroscopy
Feedback Form
Quiz: Week 12 : Assignment 12
Download Videos
Lecture Slides
Problem Solving Session
Week 12 :
Assignment 12
The due date for submitting this assignment has
passed.
Due on 2022-10-19, 23:59 IST.
Assignment submitted on
2022-10-18, 21:51 IST
1) Which of the following techniques can be 1 point
used to distinguish between the give two isomers?
I. Mass spectroscopy
13
II. C NMR
1
III. H NMR
III
II
I, II, III
None of the above
Yes, the answer is correct.
Score: 1
Accepted Answers:
I, II, III
2) Reference compound used in NMR 1 point
spectroscopy is?
Silane
Tetramethylsilane
Dimethylsilane
Trimethylsilane
Yes, the answer is correct.
Score: 1
Accepted Answers:
Tetramethylsilane
3) One of the following compounds gives 3 1 point
signals in NMR spectroscopy?
CH3–COOH
CH3 – CH2 – NH2
CH3 – O – CH3
CH3 – CH2 – Cl
Yes, the answer is correct.
Score: 1
Accepted Answers:
CH3 – CH2 – NH2
4) How many Hertz does 1 ppm corresponds 1 point
to a PMR spectrometer operating at a radio frequency
of 60 MHz and 100 MHz?
60 Hz, 100 Hz
10 Hz, 60Hz
66 Hz, 6 Hz
10 Hz, 60 Hz
Yes, the answer is correct.
Score: 1
Accepted Answers:
60 Hz, 100 Hz
5) How many signals do you expect to see in 1 point
the 1H NMR spectra of 2-Bromopropane (CH3)2CHBr
and 1-Bromopropane CH3CH2CH2Br?
2; 3
3;2
1;2
1;3
Yes, the answer is correct.
Score: 1
Accepted Answers:
2; 3
6) Mass spectrometer separates ions on the 1 point
basis of
Mass
Charge
Molecular weight
Mass to charge ratio
Yes, the answer is correct.
Score: 1
Accepted Answers:
Mass to charge ratio
7) A PMR spectrometer operates at 300MHz. 1 point
Find the value of the magnetic field. Given gN=5.585
and BN =5.05 ×10-27 JT-1
7.05 T
6.38 T
7.58 T
5093 T
Yes, the answer is correct.
Score: 1
Accepted Answers:
7.05 T
8) The mass spectrum of acetone 1 point
(CH3COCH3) shows major peaks at m/z = 58, 43 and
15. What can be deduced from these data?
The parent ion is observed, and fragmentation
involves cleavage of two C–C bonds.
The parent ion is not observed
The parent ion is observed, and fragmentation
involves loss of CO
The parent ion is observed, and fragmentation
involves cleavage of a C–C bond
Yes, the answer is correct.
Score: 1
Accepted Answers:
The parent ion is observed, and fragmentation
involves cleavage of a C–C bond
9) Which of the following cannot be used as 1 point
an analyser in MS?
Ion trap
TOF
ESI
Quadrupole
Yes, the answer is correct.
Score: 1
Accepted Answers:
ESI
10)But-2-yne has the structure H3C–C≡C– 1 point
CH3. Major peaks in the mass spectrum of but- 2-yne
appear at m/z = 54, 39 and 27. Which of the following
is inconsistent with these data?
But-2-yne fragments by loss of two methyl
groups
+ The highest mass peak corresponds to [C4H6]
But-2-yne fragments by loss of a methyl group
But-2-yne fragments by cleavage of a C–C
bond
Yes, the answer is correct.
Score: 1
Accepted Answers:
But-2-yne fragments by loss of two methyl groups
11)The mass spectrum of CH3NO2, shows 1 point
major peaks at m/z 61, 46, 30 (base peak) and 15.
Which statement is inconsistent with above data?
The parent ion is observed
The parent ion is observed
[NO]+ is a fragment ion
[NO2]+ is not formed as a fragment
No, the answer is incorrect.
Score: 0
Accepted Answers:
The parent ion is observed
12)What is δ value of aldehyde proton in 1 point
PMR spectrum?
1–2
4–5
7–8
9–10
Yes, the answer is correct.
Score: 1
Accepted Answers:
9–10
13)Correct order chemical shift of proton is? 1 point
CH3F < CH3I < CH3Br < CH3Cl
CH3I < CH3Br < CH3Cl < CH3F
CH3I < CH3F < CH3Br < CH3Cl
CH3F > CH3I > CH3Br > CH3Cl
Yes, the answer is correct.
Score: 1
Accepted Answers:
CH3I < CH3Br < CH3Cl < CH3F
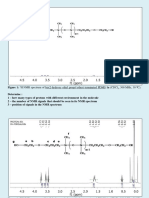
14)The 1H NMR of below given compound 1 point
should have four signals. Predict the splitting pattern
(singlet, doublet, doublet of doublets, etc) for each
signal.
Singlet, doublet, triplet, doublet
Doublet, doublet, singlet, doublet of triplet
Doublet, doublet, doublet of triplet
Singlet, doublet, triplet
No, the answer is incorrect.
Score: 0
Accepted Answers:
Doublet, doublet, singlet, doublet of triplet
15)How many peaks will be observed in the 1 point
proton NMR spectrum of (CH3)2CHCOO(CH3)?
4
5
1
3
No, the answer is incorrect.
Score: 0
Accepted Answers:
3
You might also like
- ch9 Test-BankDocument85 pagesch9 Test-Bank박훈희No ratings yet
- Validate Analytical MethodsDocument9 pagesValidate Analytical MethodsFernando Silva BetimNo ratings yet
- LatihanDocument18 pagesLatihanAhmad DulfiNo ratings yet
- Ultrasonic PrintDocument2 pagesUltrasonic PrintALNo ratings yet
- NMR Multiple Choice Questions PDFDocument71 pagesNMR Multiple Choice Questions PDFDeepak SinghNo ratings yet
- PDF - Test Bank PDF - Test BankDocument85 pagesPDF - Test Bank PDF - Test BankKathy YellaNo ratings yet
- NMR 1Document3 pagesNMR 1amitNo ratings yet
- Question Bank On Ir Spectroscopy-MatDocument10 pagesQuestion Bank On Ir Spectroscopy-MatRohan Sharma33% (3)
- CHEM20024 Lecture Notes 04 - CrystalsDocument53 pagesCHEM20024 Lecture Notes 04 - CrystalsEzriel QuantumNo ratings yet
- 1H NMR Spectroscopy in Organic Chemistry - MCQDocument18 pages1H NMR Spectroscopy in Organic Chemistry - MCQShunmugasundaram ArunachalamNo ratings yet
- Sandip University: Chemistry Cia:3 (Powerpoint Presentation) Topic: Conductometric TitrationDocument8 pagesSandip University: Chemistry Cia:3 (Powerpoint Presentation) Topic: Conductometric TitrationVidya KumariNo ratings yet
- Introduction to analytical Separations techniquesDocument43 pagesIntroduction to analytical Separations techniquesVel MuruganNo ratings yet
- Solomons Test Bank 1H NMR MCQsDocument84 pagesSolomons Test Bank 1H NMR MCQsPatrick Malcolm Santos80% (5)
- TMS Tussentoets NMR (Please Note, Questions 4, 26, 40, 42-43, 45, 47, 51, 57-60, 64, 69-75, 82-85, 92, 104-105 Were NOT Included in Your Test)Document31 pagesTMS Tussentoets NMR (Please Note, Questions 4, 26, 40, 42-43, 45, 47, 51, 57-60, 64, 69-75, 82-85, 92, 104-105 Were NOT Included in Your Test)Thatyane Kary100% (5)
- NMR Organic ChemistryDocument17 pagesNMR Organic Chemistrysallymoon34No ratings yet
- Acid-Base Titrations 2Document27 pagesAcid-Base Titrations 2John Henrick G. Uy100% (2)
- NMR - Multiple Choice QuestionsDocument71 pagesNMR - Multiple Choice QuestionsOmSilence265171% (31)
- Structure Determination of Organic Compounds - ChemvoiceDocument37 pagesStructure Determination of Organic Compounds - ChemvoiceJubin Kumar0% (1)
- Spectroscopic Techniques For Pharmaceutical and Biopharmaceutical Industries - Unit 8 - Week 7 PDFDocument1 pageSpectroscopic Techniques For Pharmaceutical and Biopharmaceutical Industries - Unit 8 - Week 7 PDFantony bevanNo ratings yet
- Spectroscopic Techniques For Pharmaceutical and Biopharmaceutical Industries - Unit 9 - Week 8 PDFDocument1 pageSpectroscopic Techniques For Pharmaceutical and Biopharmaceutical Industries - Unit 9 - Week 8 PDFantony bevanNo ratings yet
- Spectroscopic Techniques For Pharmaceutical and Biopharmaceutical Industries - Unit 6 - Week 5 PDFDocument1 pageSpectroscopic Techniques For Pharmaceutical and Biopharmaceutical Industries - Unit 6 - Week 5 PDFantony bevanNo ratings yet
- Spectroscopic Techniques For Pharmaceutical and Biopharmaceutical Industries - Unit 7 - Week 6 PDFDocument1 pageSpectroscopic Techniques For Pharmaceutical and Biopharmaceutical Industries - Unit 7 - Week 6 PDFantony bevanNo ratings yet
- Quiz 2 PDFDocument5 pagesQuiz 2 PDFahmedNo ratings yet
- Unit 5 - Week - 3: Assignment 3Document3 pagesUnit 5 - Week - 3: Assignment 3Sooraj RajanNo ratings yet
- SpectrumDocument4 pagesSpectrumBashir Dar100% (1)
- Ut Ii F 4 ADocument6 pagesUt Ii F 4 AChandrasekhar Sonar100% (1)
- Calibration of UV - Visible Spectrophotometer - Pharmaceutical GuidelinesDocument3 pagesCalibration of UV - Visible Spectrophotometer - Pharmaceutical Guidelinessureshcal131No ratings yet
- 2nd PUC Mathematics March 2016Document15 pages2nd PUC Mathematics March 2016ANUSHANo ratings yet
- Sensitivity Calibrations and TCGs ExplainedDocument20 pagesSensitivity Calibrations and TCGs ExplainedKevin HuangNo ratings yet
- XRD Particle Size PDFDocument2 pagesXRD Particle Size PDFgizachew100% (1)
- CY4104Document3 pagesCY4104Aakash BanerjeeNo ratings yet
- Cau Hi TRC Nghim Co Dap AnDocument25 pagesCau Hi TRC Nghim Co Dap AnAbdelfattah Mohamed OufNo ratings yet
- What Is The Main Source of MRI Signal? (1 PTS) : Possible AnswersDocument13 pagesWhat Is The Main Source of MRI Signal? (1 PTS) : Possible AnswersfaisalNo ratings yet
- CHM-X: Tata Institute of Fundamental ResearchDocument18 pagesCHM-X: Tata Institute of Fundamental ResearchSwatee PuhanNo ratings yet
- Assignment NMR-spectrosDocument4 pagesAssignment NMR-spectrosShovan SwainNo ratings yet
- W NMR All PDFDocument17 pagesW NMR All PDFkishor borseNo ratings yet
- Laser Midterm Test 2021 - Answer SheetDocument1 pageLaser Midterm Test 2021 - Answer SheetMahmoud AnwerNo ratings yet
- Chapter 9 Organic Chemistry SolomonDocument6 pagesChapter 9 Organic Chemistry SolomonSukhi Sohal0% (1)
- Questions & Answers: For For For For For NEET (UG) - 2020Document21 pagesQuestions & Answers: For For For For For NEET (UG) - 2020Annapurna RoutNo ratings yet
- Model Answer: The Following Questions Answer Choose The Correct Answer: (20Document4 pagesModel Answer: The Following Questions Answer Choose The Correct Answer: (20Khalid AbeedNo ratings yet
- Problemas de Espectroscopia Organica IDocument5 pagesProblemas de Espectroscopia Organica IGabriel Alejandro Socias EsquivelNo ratings yet
- NMR Spectropscopy HkaurDocument42 pagesNMR Spectropscopy Hkaurakshatgoyal643No ratings yet
- 13C NMR Spectroscopy in Organic Chemistry MCQDocument27 pages13C NMR Spectroscopy in Organic Chemistry MCQShunmugasundaram ArunachalamNo ratings yet
- Chemistry 3rd May Part TestDocument8 pagesChemistry 3rd May Part TestJaswant Singh BistNo ratings yet
- 核磁共振部分习题及答案 1Document14 pages核磁共振部分习题及答案 1Tariq Rahim MarwatNo ratings yet
- C1100E-TDDocument27 pagesC1100E-TDghadirtaleb4No ratings yet
- Old Questions AAS NMR and MSDocument7 pagesOld Questions AAS NMR and MSramesh pokhrelNo ratings yet
- MCQDocument14 pagesMCQشمس صبيح عبد الرحيم100% (1)
- MCQ For Question PaperDocument11 pagesMCQ For Question PaperAjay Sharma Shankyan100% (2)
- Ch328n Xm1 Key ColapretDocument13 pagesCh328n Xm1 Key Colapretjshortreed71100% (2)
- CHM-X: Tata Institute of Fundamental ResearchDocument12 pagesCHM-X: Tata Institute of Fundamental ResearchDebasish SharmaNo ratings yet
- 13C NMR SpectrosDocument16 pages13C NMR Spectrosapi-3723327100% (4)
- Analyzing 1H NMR Spectra of PDMSDocument5 pagesAnalyzing 1H NMR Spectra of PDMSAsrina RoslanNo ratings yet
- Unit - 2 MCQ'sDocument5 pagesUnit - 2 MCQ'srishavr2001No ratings yet
- 1 Answer:4: Quick Revision Test Integer Type QuestionsDocument41 pages1 Answer:4: Quick Revision Test Integer Type QuestionsSanthosh SenthilNo ratings yet
- CLS JEEAD-18-19 XI Che Target-4 SET-2 Chapter-12 PDFDocument42 pagesCLS JEEAD-18-19 XI Che Target-4 SET-2 Chapter-12 PDFRitik RajNo ratings yet
- Lecture 29 PDFDocument25 pagesLecture 29 PDFRachit ShahNo ratings yet
- Ochem Test 1Document11 pagesOchem Test 1careytranNo ratings yet
- Jee (A) Sample PaperDocument28 pagesJee (A) Sample PaperPrabhav PatilNo ratings yet
- Chemistry 34Document13 pagesChemistry 34Bale varun kumarNo ratings yet
- CHEM F111: General Chemistry: PilaniDocument25 pagesCHEM F111: General Chemistry: Pilanicukdbjsisns shsusbsbvzNo ratings yet
- Optimizing Phases of CPMG Pulse Sequence and Applying Exact Solution To Measure Relaxation TimeDocument29 pagesOptimizing Phases of CPMG Pulse Sequence and Applying Exact Solution To Measure Relaxation TimehellorunNo ratings yet
- MCQs For Chapter 7-12 KeyDocument11 pagesMCQs For Chapter 7-12 KeyismahijNo ratings yet
- 2021 2 CH101Document30 pages2021 2 CH101Donut NerdNo ratings yet
- Chemistry Unit 2 MCQ With AnswersDocument5 pagesChemistry Unit 2 MCQ With AnswersKaran VaswaniNo ratings yet
- Acid and Salt EquilibriaDocument26 pagesAcid and Salt EquilibriaAnthony AbesadoNo ratings yet
- Epsilon 1: Flexible and Accurate Elemental AnalysisDocument12 pagesEpsilon 1: Flexible and Accurate Elemental AnalysistonyNo ratings yet
- Standardization of Sodium Hydroxide: Experiment No. 1.2Document8 pagesStandardization of Sodium Hydroxide: Experiment No. 1.2theressaNo ratings yet
- Gel Filtration Selection GuideDocument1 pageGel Filtration Selection GuideDolphingNo ratings yet
- Instrumental Analysis Viva Voice QuestionsDocument4 pagesInstrumental Analysis Viva Voice QuestionsK.Selvaraj100% (1)
- Bmab 023Document7 pagesBmab 023Hasna NoerNo ratings yet
- Lab Report Basic Instrumental Exp 6Document7 pagesLab Report Basic Instrumental Exp 6Azli AzmanNo ratings yet
- Understanding Buffer SolutionsDocument67 pagesUnderstanding Buffer Solutionsjoshua andreNo ratings yet
- StandardizationDocument3 pagesStandardizationMuhammad ArsalanNo ratings yet
- Chem101103 Summerfinalexam SolutionDocument5 pagesChem101103 Summerfinalexam SolutionbrianNo ratings yet
- !!!! Photoconjugation of An Fc-Specific PeptideDocument30 pages!!!! Photoconjugation of An Fc-Specific PeptideJan PolachNo ratings yet
- Ostwald's Theory of Acid and Base IndicatorsDocument12 pagesOstwald's Theory of Acid and Base IndicatorsPushkar WaneNo ratings yet
- Acid-Base Titrations 2Document27 pagesAcid-Base Titrations 2Doc KhemNo ratings yet
- Natrium DiklofenakDocument3 pagesNatrium DiklofenakDestiKarmilasariNo ratings yet
- Heterogeneity tests of Chilean porphyry oresDocument12 pagesHeterogeneity tests of Chilean porphyry oresAldoNo ratings yet
- Practical 14 Iron Wool by Redox TitrationDocument4 pagesPractical 14 Iron Wool by Redox TitrationAngnes PoliskaNo ratings yet
- Development and Validation of RP-HPLC Method For Simultaneous Determination of Guaifenesin Impurities in Multi Drug CombinationsDocument9 pagesDevelopment and Validation of RP-HPLC Method For Simultaneous Determination of Guaifenesin Impurities in Multi Drug CombinationsRouag AbdelkarimNo ratings yet
- Chapter 7-2 ConductivityDocument17 pagesChapter 7-2 ConductivityNajmul Puda PappadamNo ratings yet
- Lec 2Document19 pagesLec 2Ajay Pal NattNo ratings yet
- Experiment 1: Crystallization of Impure Acetanilide ObjectiveDocument4 pagesExperiment 1: Crystallization of Impure Acetanilide ObjectiveEZLYEN AZLINNo ratings yet
- 2006-Huang & Kong (2006) Steroidal Saponins From Roots of Asparagus OfficinalisDocument6 pages2006-Huang & Kong (2006) Steroidal Saponins From Roots of Asparagus OfficinalisZulfikar0526No ratings yet
- Assignment1 110623105052 Phpapp01 PDFDocument30 pagesAssignment1 110623105052 Phpapp01 PDFahmedNo ratings yet
- Gravimetric Analysis and Precipitation - TitrationsDocument34 pagesGravimetric Analysis and Precipitation - TitrationsElvinNo ratings yet
- G7 FORENSIC INSTRUMENTATION FinalDocument34 pagesG7 FORENSIC INSTRUMENTATION FinalJason CorderoNo ratings yet
- Determination of Carbohydrates by Anion Exchange Chromatography With Pulsed Amperometric DetectionDocument16 pagesDetermination of Carbohydrates by Anion Exchange Chromatography With Pulsed Amperometric DetectionXYZUSPNo ratings yet