Professional Documents
Culture Documents
Starch Hydrolysis by Amylase: Explain Why Enzyme Activity Depend On The PH
Uploaded by
Rania Alkhozae0 ratings0% found this document useful (0 votes)
3 views2 pagesOriginal Title
1
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
3 views2 pagesStarch Hydrolysis by Amylase: Explain Why Enzyme Activity Depend On The PH
Uploaded by
Rania AlkhozaeCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 2
Starch Hydrolysis by Amylase
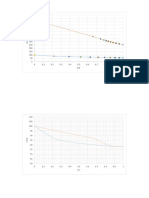
4 7 9
0.0745 0.6146 0.2637
0 Room T 100
0.0503 0.6146 0.0166
- ExplainPHwhy enzyme activity depend on the pH.Temperature Within the enzyme
0.7
molecule, positively and negatively charged amino acids will attract. ThisOptical ph
0.7
0.6 contributes to the folding of the enzyme molecule, its shape, and the shape
0.6
0.5 of the active site. Changing the pH will affect the charges on the amino acid
0.5
0.4
molecules.
0.4
0.3 0.3
0.2 0.2
0.1 0.1
0
0 0 20 40 60 80 100 120
3 4 5 6 7 8 9 10
- Discuss why do you choose this optimal temperature and pH. It is
the point at which it provides maximum activity of the enzyme the enzyme
can work best.
- Acetate pH buffer solution
Sodium Acetate (mw: 82.03 g/mol)
Acetic Acid (mw: 60.05 g/mol)
1. Prepare 800 mL of distilled water in a suitable container.
2. Add 5.772 g of Sodium Acetate to the solution.
3. Add 1.778 g of Acetic Acid to the solution.
4. Adjust solution to desired pH using 10N HCl (typically pH ≈
5.0).
5. Add distilled water until the volume is 1 L.
You might also like
- Anthrone ReagentDocument6 pagesAnthrone ReagentMitchellMarkMarmitaNo ratings yet
- BIO 462 Experiment 2Document6 pagesBIO 462 Experiment 2Nurul Farhah RadzuwanNo ratings yet
- Biochem Lab - Enzyme Activity (Corpuz, R)Document7 pagesBiochem Lab - Enzyme Activity (Corpuz, R)Reynand MaelNo ratings yet
- Lab 6Document8 pagesLab 6api-3473405070% (1)
- IA Sample 4 Students To Upload On MBDocument13 pagesIA Sample 4 Students To Upload On MBSiddhesh SoodNo ratings yet
- Experiment 7 Characterization of Serine Proteases: GBE 302 Biochemistry Ii LaboratoryDocument8 pagesExperiment 7 Characterization of Serine Proteases: GBE 302 Biochemistry Ii LaboratoryDeniz YılmazNo ratings yet
- Acid Base Indicators Lab ReportDocument6 pagesAcid Base Indicators Lab Reportmuskaan0% (2)
- Report CRE01Document7 pagesReport CRE01munazziliitdNo ratings yet
- Determining The Properties of An EnzymeDocument6 pagesDetermining The Properties of An EnzymeGeoffreiTNo ratings yet
- Bio Report (1) PrinttDocument5 pagesBio Report (1) PrinttNatalie OrtizNo ratings yet
- Marsh Funnel Test ResultDocument47 pagesMarsh Funnel Test Resultjaysern7No ratings yet
- Acetic Acid Water Isopropyl EtherDocument4 pagesAcetic Acid Water Isopropyl EtherAmalia Rizki FauziahNo ratings yet
- Diagram Vapour Liquid Equilibrium Ethanol-WaterDocument3 pagesDiagram Vapour Liquid Equilibrium Ethanol-WaterFindhita Kusuma Putri SuwitaNo ratings yet
- Etanol-Agua v.3.0Document2 pagesEtanol-Agua v.3.0Nelson Mamani LeonNo ratings yet
- Enzymes: Ust Fop - Department of BiochemistryDocument19 pagesEnzymes: Ust Fop - Department of BiochemistryAra NuesaNo ratings yet
- Cloruro FerricoDocument8 pagesCloruro FerricoMario GutiérrezNo ratings yet
- Etanol-Agua v.3.0Document2 pagesEtanol-Agua v.3.0Nelson Mamani LeonNo ratings yet
- Etanol-Agua v.3.0Document2 pagesEtanol-Agua v.3.0Nelson Mamani LeonNo ratings yet
- Etanol-Agua v.3.0Document2 pagesEtanol-Agua v.3.0Nelson Mamani LeonNo ratings yet
- Brewing MaterialsDocument1 pageBrewing MaterialsRiyanNo ratings yet
- Lab 7 Post Lab (AutoRecovered)Document5 pagesLab 7 Post Lab (AutoRecovered)Maisy BrouilletteNo ratings yet
- Effects of Enzyme Concentration On Rate of ReactionDocument2 pagesEffects of Enzyme Concentration On Rate of Reactionmigire kennedyNo ratings yet
- Jar Test ReportDocument10 pagesJar Test ReportDezzyNo ratings yet
- Chart TitleDocument3 pagesChart TitleSolange RiveraNo ratings yet
- Chemical KineticsDocument3 pagesChemical KineticsAlyssa OrtegaNo ratings yet
- Universidad Autónoma de Coahuila: Facultad de Ciencias BiológicasDocument8 pagesUniversidad Autónoma de Coahuila: Facultad de Ciencias BiológicasCuauhtémoc MedéllinNo ratings yet
- Lab Report 3 sbl1023Document5 pagesLab Report 3 sbl1023api-385038701No ratings yet
- Figure 1 Sulfolane UnitDocument2 pagesFigure 1 Sulfolane Unittusen krishNo ratings yet
- 12 SupportDocument5 pages12 SupportBé Kem Xưởng MayNo ratings yet
- Exp05 AssignmentDocument8 pagesExp05 AssignmentYara HemedaNo ratings yet
- A Note On Quantitative Urobilinogen Determinations: Bernard BalikovDocument5 pagesA Note On Quantitative Urobilinogen Determinations: Bernard BalikovTanveerNo ratings yet
- The Resulting Effect When Lactase Enzyme Is Subjected To Differences Substrate Concentration, Temperature and PHDocument6 pagesThe Resulting Effect When Lactase Enzyme Is Subjected To Differences Substrate Concentration, Temperature and PHjustusNo ratings yet
- Efectos Sobre El DNA: Temperatura ( C)Document6 pagesEfectos Sobre El DNA: Temperatura ( C)Fabian RodriguezNo ratings yet
- Tambak 1 Tambak 2: Total Rata-Rata KandunganDocument7 pagesTambak 1 Tambak 2: Total Rata-Rata KandunganUcii EmsiilNo ratings yet
- Foodlog Date 2 7 24 - Daily IntakeDocument2 pagesFoodlog Date 2 7 24 - Daily Intakeapi-732118476No ratings yet
- Report - Cation ExchangerDocument5 pagesReport - Cation ExchangerMai HoangNo ratings yet
- Sample Lab ReportDocument7 pagesSample Lab ReportdaniellaNo ratings yet
- Variabel Tugas Akhir Perovskite Solar CellDocument2 pagesVariabel Tugas Akhir Perovskite Solar CellmchchristianNo ratings yet
- Foodlog Date January 31 - Daily IntakeDocument1 pageFoodlog Date January 31 - Daily Intakeapi-732764034No ratings yet
- Ecuacion Van LaarDocument8 pagesEcuacion Van LaarAvj ParceroNo ratings yet
- Nutri Meta Lab 2 CombinedDocument16 pagesNutri Meta Lab 2 CombinedvlcjNo ratings yet
- Amylase PDFDocument1 pageAmylase PDFSenafoet NuñezNo ratings yet
- Analisis Data Bobot JenisDocument1 pageAnalisis Data Bobot JenischristiawanNo ratings yet
- Full Complete Lab Presentation (Group 6)Document31 pagesFull Complete Lab Presentation (Group 6)Aneesch PreethaNo ratings yet
- Lab Report Exp 3Document4 pagesLab Report Exp 3api-384913960No ratings yet
- How Can I Make A 4N Naoh Solution From Naoh With M 40G/Mol?: Titratable Acidity - TaDocument4 pagesHow Can I Make A 4N Naoh Solution From Naoh With M 40G/Mol?: Titratable Acidity - TaYusiNurmaNo ratings yet
- Amylase Experiment Lab ReportDocument7 pagesAmylase Experiment Lab ReportCHLOE IANNAH CALVADORESNo ratings yet
- CHE 312 - Spring 2024 - HPR - 1Document3 pagesCHE 312 - Spring 2024 - HPR - 1Yaren ErelNo ratings yet
- Physical Organic 1 Post-LabDocument7 pagesPhysical Organic 1 Post-LabsamNo ratings yet
- Experiment 3: Ternary Phase Diagram (Liquid-Liquid Extraction)Document15 pagesExperiment 3: Ternary Phase Diagram (Liquid-Liquid Extraction)Noor Nasuha Noor AriffinNo ratings yet
- A/Silicate Sada Ash SA-5Document3 pagesA/Silicate Sada Ash SA-5aleNo ratings yet
- Esterification of EthanolDocument15 pagesEsterification of EthanolSadia HasanNo ratings yet
- Spreadsheet Program For Calculating The PH - Coagulant Dosage RelationshipDocument7 pagesSpreadsheet Program For Calculating The PH - Coagulant Dosage RelationshipMohamed TallyNo ratings yet
- Vitamin B6 in Reconstituted Infant FormulaDocument3 pagesVitamin B6 in Reconstituted Infant FormulaAhmed GwealyNo ratings yet
- Figure M 39 Ethanol Water MixtureDocument6 pagesFigure M 39 Ethanol Water MixtureHamza ShafiqNo ratings yet
- Universiti Teknologi Mara: Name: Ain Athirah Binti RahimiDocument12 pagesUniversiti Teknologi Mara: Name: Ain Athirah Binti RahimiainrahimiNo ratings yet
- Milk Balance Dock1Document9 pagesMilk Balance Dock1Pritam Nale PrinsNo ratings yet
- Incompatibility in Parenteral Nutrition - 8 July 2016Document59 pagesIncompatibility in Parenteral Nutrition - 8 July 2016Rezti PratiwiNo ratings yet
- Libro1 (Version 1)Document6 pagesLibro1 (Version 1)Yeferson ParraNo ratings yet