Professional Documents
Culture Documents
Quimioterapia Tipos
Uploaded by
Jorge AguilarOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Quimioterapia Tipos
Uploaded by
Jorge AguilarCopyright:
Available Formats
SnapShot
Cancer chemotherapy
Luca Falzone1, Roberto Bordonaro2, and Massimo Libra1
1
Department of Biomedical and Biotechnological Sciences, University of Catania, 95123 Catania, Italy; ²Oncological Department, Garibaldi Hospital, 95126 Catania, Italy
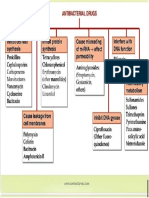
Antimetabolites Other molecules Alkylating agents Topoisomerase Other drugs
Purine and pyrimidine inhibitors Vitamins and Triazens and inhibitors Histone deacetylase
miscellaneous methylating agents Topoisomerase I and inhibitors and
topoisomerase II miscellaneous
Pyrimidine synthesis
Ribo- DNA antineoplastics
nucleotides methyltransferase Topoisomerase
U G
Cancer Deoxyribo- DNA DNA RNA Proteins Microtubules
cell Purine synthesis nucleotides polymerase Cancer cell
Antimetabolites Antimetabolites Alkylating agents Mitotic inhibitors proliferation and
Antifolates and DNA polymerase and DNA Platinum compounds, Taxanes and vinca tumor progression
miscellaneous methyltransferase inhibitors nitrogen mustards, nitrosoureas alkaloids
A L K Y L AT I N G A G E N T S A N T I M E TA B O L I T E S
Drug Mechanism of action Therapeutic applications Drug Mechanism of action Therapeutic applications
Alkylating Bladder, testicular, ovarian, head Purine Pyrimidine Colorectal, breast, stomach,
Nitrosoureas compounds
Cisplatin 1 Fluorouracil
antagonists
Pyrimidine
agents antagonists antagonists
Platinum
and neck, uterus, lung cancer pancreatic, head and neck cancer
Carboplatin Lung cancer, ovarian cancer Capecitabine Colorectal, breast and gastric
Alkyl
group cancer
Oxaliplatin Colorectal cancer
Floxuridine DNA HGPRT and Digestive system cancers
Carmustine Brain tumor, lymphoma, multiple incorporation thymidylate
antagonists
myeloma 6- synthase Acute lymphoblastic or
mercaptopurine lymphocytic leukemia
Purine
Lomustine Brain and lung tumor, malignant
RNA Acute myeloblastic or
melanoma, Hodgkin's lymphoma Thioguanine incorporation
Streptozocin Pancreatic cancer lymphoblastic leukemia
2 Nucleotide Leukemia, breast, skin, head and
Chronic lymphocytic leukemia, Methotrexate synthesis
Bendamustine Inosine neck, lung, uterine cancer
B-cell non-Hodgkin's lymphoma,
Antifolates
Antifolates
synthesis
multiple myeloma U
DNA Dihydrofolate
Non-squamous non-small cell lung
alkylation Hodgkin’s lymphoma, chronic Pemetrexed reductase cancer, malignant pleural
Chlorambucil lymphocytic leukemia, giant mesothelioma
Thymidylate G
DNA synthase
crosslink follicular lymphoma Pralatrexate T-cell lymphoma
Nitrogen mustards
Cyclo- Thymidylate Ribonucleotide
Multiple solid tumors Cladribine synthase reductase Hairy cell leukemia
phosphamide
B-cell chronic lymphocytic
Enzyme Inhibitors
Fludarabine Gemcitabine Clofarabine
Sarcoma, testicular, ovarian, leukemia
Cladribine Fludarabine
Ifosfamide bronchial, breast, pancreatic,
Cytarabine Nelarabine Pancreatic, lung, ovarian, breast
endometrial cancer, lymphoma Gemcitabine
DNA cancer
3 T-cell lymphoma, B-cell Clofarabine polymerase Acute lymphoblastic leukemia
Mechlorethamine lymphoma, chronic leukemia, lung
Multiple cancer, medulloblastoma Nelarabine T-cell lymphoblastic leukemia and
DNA strand lymphoma
breaks Multiple myeloma, ovarian cancer, Cytarabine DNA DNA Acute myeloid and other leukemias
Melphalan neuroblastoma, melanoma, crosslinks terminator
sarcoma
Dacarbazine 4 Malignant melanoma, Hodgkin's
MITOTIC INHIBITORS
Triazenes
lymphoma, sarcoma
Cell cycle
Temozolomide arrest and
death Brain tumors Drug Mechanism of action Therapeutic applications
Procarbazine Hodgkin's lymphoma Metastatic castration-resistant
Cabazitaxel
prostate cancer
Taxanes
Microtubule Breast, lung, prostate, stomach,
Docetaxel polymerization
Vinca head and neck cancer
ANTI-TUMOR ANTIBIOTICS alkaloids Breast, ovarian, lung and
(Nab)
Drug Mechanism of action Therapeutic applications pancreatic cancer, AIDS-related
Paclitaxel
Anthracyclines Kaposi's sarcoma
Daunorubicin Leukemia Microtubule
Vinblastine
degradation Hodgkin's lymphoma, testicular and
Taxanes
Several solid tumors and breast cancer, Kaposi's sarcoma
alkaloids
(Liposomal)
Vinca
Anthracyclines
Doxorubicin hematological malignancies, (Liposome) Leukemia,lymphoma,
AIDS-Kaposi's sarcoma Vincristine neuroblastoma, sarcomas
DNA
Epirubicin binding TOP2 Several solid tumors and Vinorelbine Mitosis block and cell death Lung cancer and breast cancer
hematological malignancies
Idarubicin Acute myeloid/lymphoid leukemia
Arrest of DNA TOPOISOMERASE INHIBITORS
replication
Valrubicin Bladder cancer
Drug Mechanism of action Therapeutic applications
Bleomycin
Mitomycin Squamous cell and testicular (Liposomal) TOP1 inhibitors Colorectal, small-cell lung,
Inhibitors
Irinotecan pancreatic cancer
TOP1
Bleomycin carcinoma, lymphoma, pleural
effusion
Non-Anthracyclines
Topotecan Ovarian cancer, small cell lung
cancer, cervical cancer
DNA breaks TOP2 inhibitors
Dactinomycin and crosslinks Several solid tumors
Lung, testicular and ovarian
Etoposide
cancer, lymphoma, acute myeloid
Stomach, pancreatic, breast, (VP-16)
Mitomycin-C leukemia
Inhibitors
DNA bronchial carcinoma, solid tumors
TOP2
binding TOP1 TOP2 Prostate, liver and breast cancer,
Mitoxantrone leukemia, non-Hodgkin's
Prostate cancer, leukemia,
Mitoxantrone Block of DNA lymphoma
non-Hodgkin's lymphoma, breast unfolding and
Dactinomycin cancer, hepatocellular carcinoma Teniposide replication Leukemia
Mitoxantrone
See online version for legends, references,
1816 Cell 186, April 13, 2023 © 2023 Elsevier Inc. DOI https://dx.doi.org/10.1016/j.cell.2023.02.038 and declaration of interests
SnapShot
Title
Cancer of snapshot
chemotherapy title of snapshot title of snapshot title
of
1
snapshot
Luca Falzone title, andofMassimo
, Roberto Bordonaro
1
snapshot
Libra
2
title of snapshot title1
Department of Biomedical and Biotechnological Sciences, University of Catania, 95123 Catania, Italy; ²Oncological Department, Garibaldi Hospital, 95126 Catania, Italy
Author1,2 Author,1,2 and Author1,2
Affiliation #1; ²Affiliation #2; ³Affiliation #3
1
Paragraph text
Antineoplastic drugs: Main concepts
Before the mid-1900s, the treatment of cancer was based mainly on the adoption of surgery or cautery for the eradication of non-invasive tumor lesions, while no effective
therapeutic
Section header options were available for patients with advanced or metastatic diseases. Only after the Second World War, the first active ingredients with anticancer properties
were discovered
Paragraph textand adopted for the effective treatment of tumors. Since the late ‘40s, different classes of chemotherapeutic drugs have been used alone, in combination with
other agents or in association with traditional surgery or radiation therapy.1
Despite
Section the development of novel effective strategies for the treatment of tumors, including the introduction of targeted therapy and immunotherapy, chemotherapy still plays
header
crucial roles intext
Paragraph the fight against cancer alone or in combination with other regimens.
At present, the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have approved several antineoplastic agents able to arrest tumor progression
through
Section direct
header or indirect mechanisms. 2,3 These drugs are classified into the following categories according to their mechanisms of action: alkylating agents, antimetabolites,
mitotic
Paragraph text topoisomerase inhibitors, and antitumor antibiotics plus a more heterogeneous group of agents with unknown or miscellaneous anticancer activities. Overall,
inhibitors,
chemotherapeutic drugs arrest the growth of tumors preventing the production of nucleotides, inhibiting key enzymes involved in the maintenance, duplication and transcription
of DNA, or
Section blocking proteins and cell structures fundamental for the duplication of cancer cells.
header
According
Paragraph to the most internationally recognized guidelines for the treatments of tumors,4,5 chemotherapeutic agents can be used as first-, second- or third-line treatments in
text
different tumors for curative, inductive, consolidative or maintenance purposes in association with other anticancer strategies.
Of note, the administration of chemotherapy is associated with a wide range of adverse effects mainly represented by gastrointestinal disorders (nausea, vomiting, diarrhea
ACKNOWLEDGMENTS
or constipation), bone marrow suppression, weakness, hair loss, mucositis, heart problems, secondary neoplasms, etc., which reduce the compliance of patients to treatments;
Acknowledgements
therefore, often it maytext.be necessary to administer detoxifying or supportive products, including vitamins, probiotics and proper nutrition, in order to reduce the detrimental
effects of chemotherapy.6,7
REFERENCES
As the selection of the correct agents is still debated and this topic is often overlooked in both clinical and research settings, the present SnapShot is aimed at giving a general
overview
[Author listofwith
the “surname,
key chemotherapy drugs
first/middle currently
initial” used
followed by in
“etoncology.
al.” if needed] (year in parentheses). [article title]. Journal title volume number, page–range or article number.
Example: Asp, M., Bergenstråhle, J., and Lundeberg, J. (2020). Spatially Resolved Transcriptomes—Next Generation Tools for Tissue Exploration. Bioessays 42, 1900221.
Alkylating agents
Alkylating agents are the first category of antineoplastic drugs discovered, which completely revolutionized the treatment of tumors. This group of agents contains several active
ingredients classified as nitrogen mustards, platinum compounds, nitrosoureas, triazanes, and methylating agents. Other less-used agents are alkyl sulfonates, ethyleneimines,
and methylmelamines. All these drugs can alkylate nucleic acids and proteins leading to the formation of intra- and inter-strand crosslinks responsible for multiple DNA breaks
occurring during DNA duplication. Both single- or double-strand DNA breaks are in turn associated with cell cycle arrest and cell death. These agents have different routes of
administration and are used for the treatment of hematological and solid tumors, including brain tumors by crossing the Blood-Brain Barrier.
Antimetabolites
Antimetabolites are a class of drugs whose mechanism of action is via interfering with both DNA and RNA synthesis. This category contains: purine and pyrimidine antagonists,
which are incorporated in the nascent DNA and RNA structure, thereby terminating further chain elongation or are able to interfere with enzymes involved in the production
of nitrogenous bases; antifolates, which are responsible for the inhibition of ribo- and deoxyribonucleotide synthesis; and DNA polymerase and ribonucleotide reductase
inhibitors, which can block different enzymes and to bind the DNA inducing crosslinks and chain termination. Besides these classes of antimetabolites there are also DNA
Methyltransferase inhibitors and other drugs with non-specific mechanisms of action. These drugs can be administered intravenously or orally and are used for the treatment of
almost all tumors, especially hematological tumors.
Mitotic inhibitors
Mitotic inhibitors are plant-derived agents able to induce cell cycle arrest by preventing the formation of microtubules. This category contains vinca alkaloids and taxanes;
the former can bind the tubulin of microtubules inhibiting their assembly, while the latter prevents microtubule disassembly by binding the same component. At present, both
natural and semi-synthetic drugs are available for the treatment of several solid tumors, including breast, lung, ovarian and prostate cancers as well as leukemia and lymphoma
through intravenous administration.
Anti-tumor antibiotics
Cytotoxic antibiotics comprise two different classes of drugs, anthracycline and non-anthracycline agents. The route of administration of these drugs is intravenous and
these are used for the treatment of both solid and hematological tumors. The main mechanism of action of anthracyclines is to form covalent bonds with nucleic acids and
Topoisomerase-II (TOP2), thereby interfering with DNA replication. In contrast, non-anthracyclines exert different effects; while some agents (e.g. Dactinomycin and Mitoxantrone)
directly interfere with topoisomerases, others induce DNA breaks (e.g. Bleomycin) or DNA crosslinks (e.g. Mitomycin). These activities hinder the correct replication of DNA.
Topoisomerase inhibitors
This category of drugs contains both Topoisomerase-I (TOP1) and Topoisomerase-II (TOP2) inhibitors obtained from plants. Among the first group, Irinotecan and Topotecan
bind to TOP1 to prevent DNA unwinding, which in turn inhibits DNA replication. Similarly, Etoposide and Mitoxantrone prevent DNA replication by binding TOP2. In addition, the
inhibition of Topoisomerases induces the formation of single-strand and double-strand breaks of DNA leading to the arrest of the cell cycle. These drugs can be administered
intravenously or orally for the treatment of different solid tumors and Hodgkin’s and non-Hodgkin’s lymphomas.
ACKNOWLEDGMENTS
We would like to dedicate this SnapShot to Professor Salvatore Salomone, M.D., Ph.D., for his commendable devotion to pharmacological research. He was an excellent scientist,
an outstanding mentor, and last but not least, a wonderful friend.
REFERENCES
1. Falzone, L., Salomone, S., and Libra, M. (2018). Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front Pharmacol 9, 1300.
2. EMA. Medicines: European Medicines Agency. https://www.ema.europa.eu/en/medicines/field_ema_web_categories%253Aname_%20field/Human.
3. US FDA. Oncology (Cancer) / Hematologic Malignacies Approval Notifications. https://www.fda.gov/drugs/resources-information-approved-drugs/oncology-cancer-hematologic-
malignancies-approval-notifications.
4. ESMO. Guidelines. https://esmo.org/guidelines.
5. ASCO. Guidelines, Tools, & Resources. https://old-prod.asco.org/practice-patients/guidelines.
6. Kanarek, N., Petrova, B., and Sabatini, D.M. (2020). Nature 579, 507–517.
7. McQuade, J.L., Daniel, C.R., Helmink, B.A., and Wargo, J.A. (2019). Lancet Oncol 20, e77–e91
2 Cell ???, ???
1816.e1 Cell 186,
??,April
202213,
©2022
2023 Elsevier
© 2023 Elsevier
Inc. DOI
Inc.
https://dx.doi.org/10.1016/j.cell.?????????????????
DOI https://dx.doi.org/10.1016/j.cell.2023.02.038
You might also like
- Pharm Drug ListDocument17 pagesPharm Drug Listanon_523534678No ratings yet
- Antibiotics - Compleate ClassificationDocument2 pagesAntibiotics - Compleate ClassificationNeal Gupta83% (12)
- Ultimate ChecklstDocument1 pageUltimate ChecklstMark ObiasNo ratings yet
- PHARMACOLOGY HighlightedDocument37 pagesPHARMACOLOGY HighlightedEmeroot RootNo ratings yet
- Mood Management Course - 02 - Depression Cognitive Behavioural TherapyDocument3 pagesMood Management Course - 02 - Depression Cognitive Behavioural TherapyBlanca De Miguel CabrejasNo ratings yet
- Alkylating and AntimetabolitesDocument38 pagesAlkylating and AntimetabolitesRebecca Chen0% (1)
- Cancer Chemotherapy: Firoz AnwarDocument46 pagesCancer Chemotherapy: Firoz Anwararunesh198No ratings yet
- Sample 20715Document16 pagesSample 20715Sachin KumarNo ratings yet
- Anti Cancer ChemotherapyDocument103 pagesAnti Cancer ChemotherapyMourian AmanNo ratings yet
- Cancer Chemotherapy by MaryamDocument51 pagesCancer Chemotherapy by MaryammaryamNo ratings yet
- Chemotherapy ReviewDocument6 pagesChemotherapy ReviewkemisseNo ratings yet
- AnticancerDocument27 pagesAnticancerLoreine Jane ClaritoNo ratings yet
- Cancer Foye's Principles of Medicinal Chemistry-1219-1286Document68 pagesCancer Foye's Principles of Medicinal Chemistry-1219-1286minhxuan100% (3)
- 3 - Clinical Pharmacology of ChemotherapyDocument111 pages3 - Clinical Pharmacology of ChemotherapyChinIsiangSocitoNo ratings yet
- Evelyn Handel-Pharmaceutical Care in Targeted Therapy PDFDocument49 pagesEvelyn Handel-Pharmaceutical Care in Targeted Therapy PDFaisyahNo ratings yet
- Enoxaparin FDADocument40 pagesEnoxaparin FDAImam Nur Alif Khusnudin100% (2)
- Farmacos AntineoplasicosDocument12 pagesFarmacos AntineoplasicosJanete Martinez Fernandez67% (3)
- Principles of AnesthesiaDocument60 pagesPrinciples of AnesthesiaTakale BuloNo ratings yet
- Pharmacology TableDocument9 pagesPharmacology TableMaryam KhushbakhatNo ratings yet
- Antineoplastic 2Document69 pagesAntineoplastic 2gamas deteNo ratings yet
- Anticancer Drugs: Nur PermatasariDocument67 pagesAnticancer Drugs: Nur Permatasari0910720085No ratings yet
- Chemotherapeutic FamiliesDocument6 pagesChemotherapeutic FamiliesRice CookerNo ratings yet
- Chemotherapy ToxicityDocument37 pagesChemotherapy ToxicityCarlos Eduardo Cadu100% (1)
- Drugs For Cancer TherapyDocument102 pagesDrugs For Cancer TherapyPUTRI CHRISTIANTI TELAUMBANUA 1No ratings yet
- KANKERDocument83 pagesKANKERSekar Ayu Rizky SNo ratings yet
- 1 - AntineoplasticsDocument171 pages1 - AntineoplasticsMelika SadrolashrafiNo ratings yet
- Kerja ABDocument72 pagesKerja ABFarmasi RSUD Kramat JatiNo ratings yet
- Medical ProductDocument29 pagesMedical ProductwahyuNo ratings yet
- 2 Pharmacology of ChemotherapeuticsDocument34 pages2 Pharmacology of ChemotherapeuticsTilaye GebruNo ratings yet
- Bun - Modern Assay Techniques For Cancer DrugsDocument19 pagesBun - Modern Assay Techniques For Cancer DrugssorinamotocNo ratings yet
- Road To MDDocument1 pageRoad To MDEcel AggasidNo ratings yet
- Antimicrobal Drugs #Dental 1Document30 pagesAntimicrobal Drugs #Dental 1ggNo ratings yet
- KU PPB 423 Lesson 2 - Introduction To Antineoplastic AgentsDocument9 pagesKU PPB 423 Lesson 2 - Introduction To Antineoplastic AgentsPharmswipe KenyaNo ratings yet
- Anti Cancer DrugsDocument3 pagesAnti Cancer DrugsDheaNo ratings yet
- AntineoplasticsDocument4 pagesAntineoplasticsahmed montaserNo ratings yet
- Anticancer Drugs: Pharm 3620 Human Pharmacology and Therapeutic PrinciplesDocument24 pagesAnticancer Drugs: Pharm 3620 Human Pharmacology and Therapeutic Principleszj5bnxbymzNo ratings yet
- Cancer Chemotherapy (Cytostatics)Document41 pagesCancer Chemotherapy (Cytostatics)Raka1793No ratings yet
- Antiparasitic & Antifungal DrugsDocument30 pagesAntiparasitic & Antifungal DrugsAbdullah AlkharsNo ratings yet
- Unit 5Document81 pagesUnit 5Shraddha PaliwalNo ratings yet
- B Princ of Antimicrobial & Antineoplastic PharDocument25 pagesB Princ of Antimicrobial & Antineoplastic PharsacredgamesNo ratings yet
- Anticancer Drugs: - Visit For More Ppt'sDocument45 pagesAnticancer Drugs: - Visit For More Ppt'sGANESH KUMAR JELLANo ratings yet
- Anti Cancer Drugs - UpdatedDocument1 pageAnti Cancer Drugs - UpdatedaucukagapeNo ratings yet
- Therapi Antibiotik 1Document7 pagesTherapi Antibiotik 1Muhammad RaflyNo ratings yet
- CancerDocument17 pagesCancerZyreeneNicoleNo ratings yet
- Antineoplastic AntiCancer-DrugsDocument35 pagesAntineoplastic AntiCancer-Drugsjoyce.feir03No ratings yet
- Adobe Scan 24 Dec 2022Document1 pageAdobe Scan 24 Dec 2022Jatin ChaudharyNo ratings yet
- Aula 7 FBMCCR 2324Document16 pagesAula 7 FBMCCR 2324VanessaMarquesNo ratings yet
- Antimetabolites: Antimetabolites Is A Broad Term, and Could Potentially Refer ToDocument14 pagesAntimetabolites: Antimetabolites Is A Broad Term, and Could Potentially Refer ToAbdelrhman AboodaNo ratings yet
- Antineoplastic DrugsDocument83 pagesAntineoplastic DrugsАббасси МокаммедNo ratings yet
- Curs VII - Terapii SistemiceDocument229 pagesCurs VII - Terapii SistemiceHucay EduardNo ratings yet
- Cancer ChemotherapyDocument37 pagesCancer ChemotherapyRubyrose TagumNo ratings yet
- Principles of Systemic TreatmentDocument14 pagesPrinciples of Systemic TreatmentAna Castro MondacaNo ratings yet
- Class/ Name Indication MOA Adverse/Side Effects Notes Phase Specific Vinca AlkaloidsDocument4 pagesClass/ Name Indication MOA Adverse/Side Effects Notes Phase Specific Vinca AlkaloidsCha GabrielNo ratings yet
- 21 Anti-NeoplasticDocument11 pages21 Anti-NeoplasticMuhammad Amin BozdarNo ratings yet
- Neoplasia: Tumor Suppressor Genes: Insensitivity To Growth InhibitionDocument37 pagesNeoplasia: Tumor Suppressor Genes: Insensitivity To Growth InhibitionCiullaeNo ratings yet
- Granules Speciality PDFDocument2 pagesGranules Speciality PDFMadhavi VutukuruNo ratings yet
- Mitchell-2001-British Journal of Clinical Pharmacology PDFDocument10 pagesMitchell-2001-British Journal of Clinical Pharmacology PDFSyahrun MubarakNo ratings yet
- ANTII CANCER DRUGS LectureDocument66 pagesANTII CANCER DRUGS Lecturebakhtawar shaikhNo ratings yet
- Anticancer AgentsDocument22 pagesAnticancer AgentsDr AUNo ratings yet
- Curs VII - Terapii SistemiceDocument229 pagesCurs VII - Terapii SistemiceHucay EduardNo ratings yet
- Anticancer 1 P2Document4 pagesAnticancer 1 P2N Gv FcNo ratings yet
- Kemo AnestesiDocument11 pagesKemo AnestesiHeru Fajar SyaputraNo ratings yet
- Chlorpropamide: Drug Information: Brand Names: CanadaDocument13 pagesChlorpropamide: Drug Information: Brand Names: CanadaAnonymous wmF9p2ejNo ratings yet
- 机械循环支持治疗心力衰竭的研究进展 尤涛Document5 pages机械循环支持治疗心力衰竭的研究进展 尤涛Shimily LiangNo ratings yet
- Cochrane: LibraryDocument70 pagesCochrane: Libraryskola onlajnNo ratings yet
- Emergency Drugs in DentistryDocument4 pagesEmergency Drugs in DentistryAnusha KurianNo ratings yet
- Antimicrobial Stewardship in Primary CareDocument6 pagesAntimicrobial Stewardship in Primary CareMohammed HaiderNo ratings yet
- Formulation and Evaluation of Domperidone Candy LozengesDocument9 pagesFormulation and Evaluation of Domperidone Candy LozengeskbnarkhedeNo ratings yet
- 7.laporan STP 2 (Ok)Document47 pages7.laporan STP 2 (Ok)manda belaNo ratings yet
- Final - Narcotic Drugs and Psychotropic Substance Usage in HospitalDocument23 pagesFinal - Narcotic Drugs and Psychotropic Substance Usage in HospitalJAGDISH SAININo ratings yet
- CapecitabineDocument8 pagesCapecitabineLisa MarieNo ratings yet
- A Review On Drug Approval in Regulated and Non-Regulated MarketsDocument5 pagesA Review On Drug Approval in Regulated and Non-Regulated MarketsJohannes SchufiNo ratings yet
- Stokon Vs Detil PenjualanDocument240 pagesStokon Vs Detil Penjualanasthenia8No ratings yet
- Kuliah 2 Dermato Terapi 2015Document47 pagesKuliah 2 Dermato Terapi 2015Indit Septi PamujiNo ratings yet
- ImmunosuppressionDocument2 pagesImmunosuppressionSnapeSnapeNo ratings yet
- MF Mmi PDFDocument4 pagesMF Mmi PDFAbou Tebba SamNo ratings yet
- Freiburg Flap ApplicatorDocument3 pagesFreiburg Flap Applicatorapi-299403846No ratings yet
- Methanol Poisoning at A Glance PDFDocument2 pagesMethanol Poisoning at A Glance PDFAmeer MattaNo ratings yet
- Midazolam Injection: New Zealand Data SheetDocument14 pagesMidazolam Injection: New Zealand Data SheetAlin AdelineNo ratings yet
- Drug Therapy ProblemsDocument15 pagesDrug Therapy ProblemsTristanNo ratings yet
- Markowitz Supportive TX Focus 2014Document6 pagesMarkowitz Supportive TX Focus 2014DrAkhilesh SharmaNo ratings yet
- Staff Development Program - NebulizationDocument3 pagesStaff Development Program - NebulizationRene John FranciscoNo ratings yet
- Parenteral Catheterization Enema ChecklistDocument8 pagesParenteral Catheterization Enema ChecklistJessoliver GalvezNo ratings yet
- Royal Brisbane & Women's Hospital Medical Emergency / Arrest Observation and Audit FormDocument2 pagesRoyal Brisbane & Women's Hospital Medical Emergency / Arrest Observation and Audit FormYoussef MokdadNo ratings yet
- Data Farklin M.faisal PDFDocument43 pagesData Farklin M.faisal PDFfarklin rsuamiraNo ratings yet
- BCS Classification of Some Drugs:: S/No Medicine Strength Solubility Permeability ClassDocument6 pagesBCS Classification of Some Drugs:: S/No Medicine Strength Solubility Permeability Classmuhammad shoaibNo ratings yet
- RanitidineDocument1 pageRanitidineAyeNo ratings yet
- KlindexDocument2 pagesKlindexPatricia MaglasangNo ratings yet