Professional Documents
Culture Documents
Result For Tubular Reactor
Uploaded by
Irabor Emmanuel Kelvin0 ratings0% found this document useful (0 votes)
15 views1 pageOriginal Title
Result for Tubular Reactor
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
15 views1 pageResult For Tubular Reactor
Uploaded by
Irabor Emmanuel KelvinCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 1
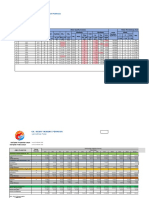
CHE 411 (EXPERIMENT 5)
RESULTS FOR DETERMINATION OF RATE CONSTANT USING A TUBULAR
REACTOR
ELAPSED TEMPERATURE CONCENTRATION IN FLOW RATE MEASURED
TIME (S) OF REACTOR TANK (MOL/DM3) (CM3/MIN) CONDUCTIVITY
(⁰C) (MS/CM)
SODIUM ETHYL HYDROXIDE ETHYL
HYDROXIDE ACETATE (Fa) ACETATE
aμ bμ (Fb)
0 26.4 0.050 0.050 50.0 0.0 0.05
30 26.5 0.050 0.050 50.0 150.0 0.04
60 26.5 0.050 0.050 131.0 150.0 0.04
90 26.5 0.050 0.050 150.0 150.0 0.04
120 26.5 0.050 0.050 150.0 150.0 0.06
150 26.5 0.050 0.050 150.0 150.0 1.48
180 26.6 0.050 0.050 150.0 150.0 2.65
210 26.5 0.050 0.050 150.0 150.0 2.75
240 26.8 0.050 0.050 150.0 150.0 2.74
270 26.9 0.050 0.050 150.0 150.0 2.72
300 27.1 0.050 0.050 150.0 150.0 2.75
330 27.2 0.050 0.050 150.0 150.0 2.79
360 27.1 0.050 0.050 150.0 150.0 2.80
390 27.1 0.050 0.050 150.0 150.0 2.80
420 27.1 0.050 0.050 150.0 150.0 2.79
450 27.0 0.050 0.050 150.0 150.0 2.84
The aims of this experiment include:
1. Determination of reaction rate constant.
2. Effect of varying the temperature on reaction rate.
3. Effect of varying the reactant concentration on reaction rate.
4. Effect of varying the feed rate and effect of flow rate on conversion.
CHE 400L CR
TOCHUKWU HENRY AMUCHIE
You might also like
- PFR Lab Analysis Determines Rate & Equilibrium ConstantsDocument11 pagesPFR Lab Analysis Determines Rate & Equilibrium ConstantsQuang TranNo ratings yet
- ANGLEDocument3 pagesANGLEskorut100% (2)
- Motor Starter Ready ReckonerDocument2 pagesMotor Starter Ready ReckoneranandpurushNo ratings yet
- DC Voltage Drop CalcDocument12 pagesDC Voltage Drop CalcJonathan Brylle CardinalNo ratings yet
- CSTR Data Sets 1 - 3Document11 pagesCSTR Data Sets 1 - 3brittanyNo ratings yet
- Hydraulic Calculation - Sprinkler System (V 1.2) - OKDocument5 pagesHydraulic Calculation - Sprinkler System (V 1.2) - OKPerunding LNB100% (1)
- Physical 2Document7 pagesPhysical 2Victor MbowuraNo ratings yet
- Measuring Rates of A Neutralization ReactionDocument8 pagesMeasuring Rates of A Neutralization ReactionElhana DyckNo ratings yet
- R Lab 1Document11 pagesR Lab 1Ahoud AlhaimliNo ratings yet
- Physical Organic 1 Post-LabDocument7 pagesPhysical Organic 1 Post-LabsamNo ratings yet
- Sky Textiles ETP RO MEE Cost ReportDocument1 pageSky Textiles ETP RO MEE Cost Reportsky textiles300No ratings yet
- Acid-Base Indicators Spectrophotometric Ka LabDocument6 pagesAcid-Base Indicators Spectrophotometric Ka Labmuskaan0% (2)
- Rate of reaction of sodium hydroxide and ethyl acetateDocument5 pagesRate of reaction of sodium hydroxide and ethyl acetateKelly Sheine SisonNo ratings yet
- Sample Number Sample Time Elapsed Time Sodium Hydroxide Concentaion (Mol/dm ) Ethyl Acetate Concentraio N (Mol/dm )Document5 pagesSample Number Sample Time Elapsed Time Sodium Hydroxide Concentaion (Mol/dm ) Ethyl Acetate Concentraio N (Mol/dm )Kelly Sheine SisonNo ratings yet
- Paket Lokasi Lataston Lapis Aus HRS-WCDocument12 pagesPaket Lokasi Lataston Lapis Aus HRS-WCDoni HidayatNo ratings yet
- HVWS System DesignDocument21 pagesHVWS System DesignSuhasNo ratings yet
- Sample Number Sample Time Elapsed Time Sodium Hydroxide Concentaion (Mol/dm ) Ethyl Acetate Concentr Aion (Mol/dm )Document4 pagesSample Number Sample Time Elapsed Time Sodium Hydroxide Concentaion (Mol/dm ) Ethyl Acetate Concentr Aion (Mol/dm )Kelly Sheine SisonNo ratings yet
- SEO-OPTIMIZED SUPPLEMENTARY TABLESDocument7 pagesSEO-OPTIMIZED SUPPLEMENTARY TABLESRAJESH SIMHADRINo ratings yet
- Q M N K: Tabel.1 Karakteristik SaluranDocument6 pagesQ M N K: Tabel.1 Karakteristik SaluranAan FachriNo ratings yet
- Chart Shows Soil Infiltration Rates Over TimeDocument82 pagesChart Shows Soil Infiltration Rates Over TimeAndi AsriadiNo ratings yet
- Network Check ACS880-04-585A-3: Network and Transformer Data Supply Unit DataDocument2 pagesNetwork Check ACS880-04-585A-3: Network and Transformer Data Supply Unit DataKrishna JashaNo ratings yet
- Clasificacion Geomecanica DDH-P-06 RVDocument6 pagesClasificacion Geomecanica DDH-P-06 RVjuanNo ratings yet
- Isolated Footing: Fy DF 15 415 130 FOT Pu Mux Muy BC DC Req. A (KN) (KN-M) (KN-M) (MM) (MM) (FT) (FT) (M) (M) F PDocument7 pagesIsolated Footing: Fy DF 15 415 130 FOT Pu Mux Muy BC DC Req. A (KN) (KN-M) (KN-M) (MM) (MM) (FT) (FT) (M) (M) F Prockey shresthaNo ratings yet
- ISOLATED FOOTING DETAILSDocument7 pagesISOLATED FOOTING DETAILSSakar ShresthaNo ratings yet
- Parameter CalculatorDocument6 pagesParameter CalculatorAnkit GuptaNo ratings yet
- Material requirements for steel productionDocument6 pagesMaterial requirements for steel productionAnonymous hj68hZmoNo ratings yet
- CEM Continuous Stirred Tank Reactor - Run 1 ResultsDocument20 pagesCEM Continuous Stirred Tank Reactor - Run 1 ResultsMartha AlbaNo ratings yet
- Esterification of EthanolDocument15 pagesEsterification of EthanolSadia HasanNo ratings yet
- Compressed Air Pressure Drop ChartDocument6 pagesCompressed Air Pressure Drop ChartWael WaelNo ratings yet
- Carta de Conversion Metrica Vs AWG CablesDocument1 pageCarta de Conversion Metrica Vs AWG CablesetorinNo ratings yet
- SULPHURIC ACID SIMULATION PART 2_PDFDocument3 pagesSULPHURIC ACID SIMULATION PART 2_PDFachik realNo ratings yet
- Pressure DropDocument6 pagesPressure Dropdept.processnamoNo ratings yet
- Deltah E K0 R Dtadiabático CP C1 0 C2 0 PDocument2 pagesDeltah E K0 R Dtadiabático CP C1 0 C2 0 PJhonNo ratings yet
- BLOK A4 (28 Oktober 2022)Document2 pagesBLOK A4 (28 Oktober 2022)Erika Nur HalimahNo ratings yet
- Flowsheet FinalDocument1 pageFlowsheet FinalYaraKanawatiNo ratings yet
- Reactive MaintenanceDocument8 pagesReactive MaintenanceAashutosh KarnaNo ratings yet
- Dimensionamento de MotobombaDocument10 pagesDimensionamento de MotobombaJoão Carlos CavalcanteNo ratings yet
- Información Del Perfil de Carga Del MotorDocument1 pageInformación Del Perfil de Carga Del MotorWalter YllahuamanNo ratings yet
- Hydro BonjeanDocument34 pagesHydro Bonjeanarqhab walzthyNo ratings yet
- Analisa Saringan Halus-1Document5 pagesAnalisa Saringan Halus-1Indriya AzuarNo ratings yet
- Traveled Way Width: 6.6 MetersDocument10 pagesTraveled Way Width: 6.6 MetersRichard Dean SantosNo ratings yet
- MT ARMADA NUGRAHA DATADocument19 pagesMT ARMADA NUGRAHA DATArizkiNo ratings yet
- HT Xlpe PDFDocument3 pagesHT Xlpe PDFrengaramanujanNo ratings yet
- Full Load Amp Table PDFDocument2 pagesFull Load Amp Table PDFBlaidimir Israel LeonNo ratings yet
- Full Load Amp Table PDFDocument2 pagesFull Load Amp Table PDFdiegoNo ratings yet
- Angle: - 45°, Frequency Range: (100 HZ, 4000 HZ)Document1 pageAngle: - 45°, Frequency Range: (100 HZ, 4000 HZ)oscaracostaNo ratings yet
- Laporan Penatalaksanaan Vaksin Upt Puskesmas Nglipar 1: Jmlah Sisa TH 2013Document2 pagesLaporan Penatalaksanaan Vaksin Upt Puskesmas Nglipar 1: Jmlah Sisa TH 2013asmiyatunNo ratings yet
- Mix Design Beton 7504Document8 pagesMix Design Beton 7504Fadli DwintaraNo ratings yet
- Vdocuments - MX - Pec Ac Resistance Reactance TableDocument2 pagesVdocuments - MX - Pec Ac Resistance Reactance TableJhoanna CalloNo ratings yet
- Electrical Design Analysis Voltage Drop Three Phase /single PhaseDocument1 pageElectrical Design Analysis Voltage Drop Three Phase /single PhaseRobert R. TiinNo ratings yet
- Miguel Quispe MolloDocument5 pagesMiguel Quispe Mollomaruja olveaNo ratings yet
- RTD Cobined PFR CSTRDocument5 pagesRTD Cobined PFR CSTRhanamant jamadarNo ratings yet
- StreamDocument4 pagesStreamNARAYAN DESAINo ratings yet
- Beer brewing process chemicals and raw materials guideDocument1 pageBeer brewing process chemicals and raw materials guideRiyanNo ratings yet
- planillademetrados arq dilverDocument4 pagesplanillademetrados arq dilverJoel MamaniNo ratings yet
- 1a. NBC, IS and ASCE Response Curve ComparisonDocument2 pages1a. NBC, IS and ASCE Response Curve ComparisonPrakash Singh RawalNo ratings yet
- Pressure Vacuum: Pressure: Pressure Gage Vs Transducer Vacuum: Mercury Manometer Vs Vacuum GageDocument4 pagesPressure Vacuum: Pressure: Pressure Gage Vs Transducer Vacuum: Mercury Manometer Vs Vacuum GageIzzah HzmhNo ratings yet
- Anthony M. Wachinski - Environmental Ion Exchange - Principles and Design-Taylor & Francis, Chapman and Hall - CRC (2016) (1) (138-157)Document20 pagesAnthony M. Wachinski - Environmental Ion Exchange - Principles and Design-Taylor & Francis, Chapman and Hall - CRC (2016) (1) (138-157)HARDY EDDISONNo ratings yet
- Stoichiometric Air CalculationsDocument5 pagesStoichiometric Air CalculationsaravindharajanNo ratings yet
- Enhanced Oil Recovery: Resonance Macro- and Micro-Mechanics of Petroleum ReservoirsFrom EverandEnhanced Oil Recovery: Resonance Macro- and Micro-Mechanics of Petroleum ReservoirsRating: 5 out of 5 stars5/5 (1)
- Extraction of Laudary Starch From CassavaDocument2 pagesExtraction of Laudary Starch From CassavaIrabor Emmanuel KelvinNo ratings yet
- 2020-21 Harmattan Lecture TimetableDocument2 pages2020-21 Harmattan Lecture TimetableIrabor Emmanuel KelvinNo ratings yet
- Top 20 Things To Do Before ReadingDocument6 pagesTop 20 Things To Do Before ReadingIrabor Emmanuel KelvinNo ratings yet
- Trend Vs No TrendDocument3 pagesTrend Vs No Trendapi-3806115No ratings yet
- Quaternary Science ReviewsDocument19 pagesQuaternary Science ReviewsFernando Nicolas Ureta GodoyNo ratings yet
- ME-51016, Fluid Mechanics II (First Semester) : Ministry of Science and Technology Technological University (Thanlyin)Document19 pagesME-51016, Fluid Mechanics II (First Semester) : Ministry of Science and Technology Technological University (Thanlyin)rob inNo ratings yet
- ANEXO Quotation Pipe AccesoriesDocument2 pagesANEXO Quotation Pipe AccesoriesJavierEnriquePalominoMendozaNo ratings yet
- Thermal Conductivity of Brass and PLADocument10 pagesThermal Conductivity of Brass and PLAJonathan WilliamsNo ratings yet
- Tri-Bore PVDF Hollow Fibers With A Super-Hydrophobic Coating For Membrane DistillationDocument11 pagesTri-Bore PVDF Hollow Fibers With A Super-Hydrophobic Coating For Membrane DistillationKellyNo ratings yet
- 235 Practice Exam 1Document11 pages235 Practice Exam 1bamforNo ratings yet
- Elliott TC - Installation, Operation & Maintenance ManualDocument37 pagesElliott TC - Installation, Operation & Maintenance ManualBlack Heart Shoaib Zaidi100% (3)
- Hogan 1998 0186Document4 pagesHogan 1998 0186Particle Beam Physics LabNo ratings yet
- Petronas Twin Tower, Kuala Lumpur, Malaysia: By-Nawal Kishor Dwivedi M.Tech Structural Engineering, MNIT JaipurDocument27 pagesPetronas Twin Tower, Kuala Lumpur, Malaysia: By-Nawal Kishor Dwivedi M.Tech Structural Engineering, MNIT Jaipurnurul jannahNo ratings yet
- Physics in Daily LifeDocument11 pagesPhysics in Daily LifeHarpuneet Kaur100% (1)
- Preventative Maintenance ChecklistDocument1 pagePreventative Maintenance ChecklistRonny ChipeNo ratings yet
- 2004 Indian Ocean Earthquake and TsunamiDocument38 pages2004 Indian Ocean Earthquake and TsunaminicasioaquinoNo ratings yet
- Elder 1967Document22 pagesElder 1967ALEJANDRO GANCEDO TORALNo ratings yet
- Chapter 02Document16 pagesChapter 02Shintaro BalingataNo ratings yet
- Single Effect EvaporatorDocument5 pagesSingle Effect Evaporatorprakashom01880% (5)
- Tic Tac Toe Choice BoardDocument2 pagesTic Tac Toe Choice Boardapi-529100008No ratings yet
- Hooke's LawDocument19 pagesHooke's LawAsyraaf YapNo ratings yet
- 28Document24 pages28Rogelio Arellano LawayanNo ratings yet
- 1 Lean Manufacturing 1.1: Lean, Is A Production Practice That ConsidersDocument37 pages1 Lean Manufacturing 1.1: Lean, Is A Production Practice That ConsidersJuanRodríguezNo ratings yet
- Earth Pressure at Rest Rankine's Theory of Earth PressureDocument27 pagesEarth Pressure at Rest Rankine's Theory of Earth PressureDrSuman ManandharNo ratings yet
- Measurement & ControlDocument5 pagesMeasurement & ControlAsif PatelNo ratings yet
- Report On Industrial Visit To HMC-1 Taxila: Mechanical Works Foundry & Forge WorksDocument7 pagesReport On Industrial Visit To HMC-1 Taxila: Mechanical Works Foundry & Forge Worksumair afzalNo ratings yet
- Catalogo de CouplingsDocument32 pagesCatalogo de CouplingsjoravicaNo ratings yet
- 317114162-EDDY-CURRENT-AND-APPLICATIONS-PROJECT Class 12 Physics Investigatory ProjectDocument9 pages317114162-EDDY-CURRENT-AND-APPLICATIONS-PROJECT Class 12 Physics Investigatory Projectpseudo network67% (9)
- PLA Hydrolysis Lab ReportDocument10 pagesPLA Hydrolysis Lab Report刘哲宁No ratings yet
- Gasket EngineeringDocument28 pagesGasket EngineeringRohit DaveNo ratings yet
- Country's Best Online Test PlatformDocument63 pagesCountry's Best Online Test PlatformSubhrasankar RaychaudhuryNo ratings yet
- Cat 3Document13 pagesCat 3Sebastian LopezNo ratings yet