Professional Documents
Culture Documents
LIGands
Uploaded by
1NC21IS038 POLATHALA MOUNIKACopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
LIGands
Uploaded by
1NC21IS038 POLATHALA MOUNIKACopyright:
Available Formats
21BE45 Biology for Engineers, Common to All 4th Semester Branches of VTU, Karnataka
Dr. Prasad Puthiyillam YouTube Channel: @ myintuition4865
Chapter 3
HUMAN ORGAN SYSTEMS AND BIO-DESIGNS - 2
3.3 Muscular Systems as Scaffolds: https://youtu.be/FV5B653B2_k
The use of muscular systems as scaffolds in regenerative medicine is an area of active
research and development. Muscles have the potential to be used as scaffolds for the
regeneration of tissues due to their inherent mechanical properties and ability to support cell
growth and tissue formation.
One example of using muscular systems as scaffolds is in the treatment of damaged or
diseased heart tissue. Researchers have developed methods for using muscle cells to create a
functional, three-dimensional scaffold that can support the growth of new heart tissue. In this
approach, muscle cells are harvested from the patient and then seeded onto a scaffold, such as a
hydrogel or artificial matrix. The scaffold provides a framework for the cells to grow and
differentiate into new heart tissue, which can help to repair the damaged or diseased tissue.
Another example is in the treatment of skeletal muscle injuries, such as those caused by
trauma or disease. In this case, muscle cells can be harvested and seeded onto a scaffold, which
can then be implanted into the damaged muscle to promote the growth of new, functional tissue.
While the use of muscular systems as scaffolds is still in the experimental stage, it holds
great promise for the treatment of a variety of conditions and represents an area of active
research and development in the field of regenerative medicine.
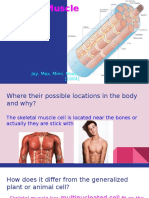
3.3.1 Architecture
Figure: The Three Connective Tissue Layers: Bundles of muscle fibers, called fascicles, are
covered by the perimysium. Muscle fibers are covered by the endomysium.
Inside each skeletal muscle, muscle fibers are organized into bundles, called fascicles,
surrounded by a middle layer of connective tissue called the perimysium. This fascicular
1 / Whatsapp +91 7975054865
21BE45 Biology for Engineers, Common to All 4th Semester Branches of VTU, Karnataka
Dr. Prasad Puthiyillam YouTube Channel: @ myintuition4865
organization is common in muscles of the limbs; it allows the nervous system to trigger a
specific movement of a muscle by activating a subset of muscle fibers within a fascicle of the
muscle. Inside each fascicle, each muscle fiber is encased in a thin connective tissue layer of
collagen and reticular fibers called the endomysium. The endomysium surrounds the
extracellular matrix of the cells and plays a role in transferring force produced by the muscle
fibers to the tendons.
Inside the muscle fibers, there are tiny structures called myofibrils. Myofibrils are made
up of smaller units called sarcomeres, which are responsible for muscle contraction.
Figure: Representing the sacromere
Sarcomeres contain thin (Actin) and thick filaments (Myosin) that work together to make
the muscle fibers contract. Each muscle fiber is surrounded by a protective layer called
endomysium. Multiple muscle fibers are grouped together into bundles called fascicles. Fascicles
are surrounded by another layer of connective tissue called perimysium.
All the fascicles together make up the entire muscle, which is surrounded by a layer
called epimysium. The muscle also has a special membrane called the sarcolemma, which
protects the muscle fiber. Inside the muscle fiber, there are small tunnels called T-tubules that
help transmit signals for muscle contraction. Muscles work through the coordination of motor
units, which consist of a motor neuron and the muscle fibers it controls. This architecture allows
muscles to generate force, move our bodies, and perform various activities.
3.3.2 Mechanisms
The mechanism of how the muscular system can be used as a scaffold in regenerative
medicine involves the use of muscle cells and a scaffold to support the growth and regeneration
of new tissue.
The method of growing muscle tissue using hydrogel or artificial scaffold is explained
below:
2 / Whatsapp +91 7975054865
21BE45 Biology for Engineers, Common to All 4th Semester Branches of VTU, Karnataka
Dr. Prasad Puthiyillam YouTube Channel: @ myintuition4865
Figure: Representing the muscle tissue growth using hydrogel or artificial scaffold
Figure: Representing the formation of polymer based scaffold and cell culture
The basic steps in this process are as follows:
3 / Whatsapp +91 7975054865
21BE45 Biology for Engineers, Common to All 4th Semester Branches of VTU, Karnataka
Dr. Prasad Puthiyillam YouTube Channel: @ myintuition4865
Harvesting of muscle cells: Muscle cells are typically obtained from the patient and then
isolated and expanded in culture.
Seeding onto scaffold: The muscle cells are then seeded onto a scaffold, such as a
hydrogel or artificial matrix. The scaffold provides a framework for the cells to grow and
differentiate into new tissue.
Cell differentiation and tissue formation: Once the cells are seeded onto the scaffold, they
undergo differentiation, in which they change into specific cell types, such as muscle
cells or heart cells. The cells also begin to organize and form new tissue, such as heart
tissue or skeletal muscle tissue.
Implantation into patient: The scaffold and cells are then implanted into the patient to
promote the growth of new, functional tissue.
3.3.3 Muscle Cells as Scaffold
Muscle cells can be used as a scaffold for tissue generation by removing the living cells
from the muscle tissue, leaving behind the structure known as the extracellular matrix (ECM).
This decellularized muscle scaffold provides a framework that can guide and support the growth
of new tissues.
Figure: Representing muscle scaffold for tissue growth
The Process
Harvesting muscle tissue: A small sample of muscle tissue is taken, typically from a
donor or an animal model.
Cell removal: The living cells within the muscle tissue are removed using a process
called decellularization. This involves treating the tissue with specific chemical solutions
4 / Whatsapp +91 7975054865
21BE45 Biology for Engineers, Common to All 4th Semester Branches of VTU, Karnataka
Dr. Prasad Puthiyillam YouTube Channel: @ myintuition4865
or enzymes that break down and wash away the cellular components, while preserving
the ECM.
ECM scaffold: The remaining ECM, which forms the structure of the muscle, is now a
scaffold. It consists of proteins, such as collagen and elastin, and other molecules that
provide support and signals for tissue growth.
Seeding cells: The decellularized muscle scaffold is then seeded with desired cells. These
can be stem cells or specialized cells relevant to the type of tissue being regenerated. The
cells are introduced onto the scaffold, allowing them to attach and populate the structure.
Tissue growth: Over time, the seeded cells proliferate and differentiate, meaning they
multiply and transform into specific cell types required for the desired tissue. The ECM
scaffold guides the cells' growth, providing physical support, and biochemical cues to
influence their behavior.
Tissue integration: As the cells continue to grow, they populate the scaffold and form
new tissue. The new tissue integrates with the surrounding native tissue, gradually
replacing the decellularized scaffold with functional, regenerated tissue.
By utilizing the decellularized muscle scaffold, the process of tissue generation takes
advantage of the existing three-dimensional architecture and mechanical properties of the
muscle. This approach has the potential to address challenges in tissue engineering, such as
creating a suitable environment for cell growth, promoting vascularization, and facilitating
functional integration of regenerated tissues.
3.3.4 Bioengineering Solutions for Muscular Dystrophy
Muscular dystrophy
Figure: Representing normal muscle and muscular dystrophy
Muscular dystrophy is a group of genetic disorders that result in progressive weakness
and degeneration of the skeletal muscles, which are responsible for movement. The disorders are
caused by mutations in genes that encode proteins needed for muscle function. The most
common type of muscular dystrophy is Duchenne muscular dystrophy, which typically affects
young boys and leads to severe disability by early adulthood. Other forms of the disease include
Becker muscular dystrophy, limb-girdle muscular dystrophy, and facioscapulohumeral
dystrophy, among others.
5 / Whatsapp +91 7975054865
21BE45 Biology for Engineers, Common to All 4 th Semester Branches of VTU, Karnataka
Dr. Prasad Puthiyillam YouTube Channel: @ myintuition4865
Duchenne muscular dystrophy (DMD) usually appears early in childhood between the
ages of 2 and 3. DMD primarily affects boys but can affect girls in rare cases. The primary
symptom of DMD is muscle weakness that begins in the muscles close to the body and later
affects muscles in the outer limbs.
Becker muscular dystrophy typically becomes apparent between the ages of 5 and 15. It
is similar to Duchenne MD, except that it progresses slower and symptoms begin to appear later.
Boys are primarily affected by Becker MD. Becker MD causes muscle loss that begins in the
hips and pelvic area, thighs, and shoulders.
The age of onset of limb-girdle muscular dystrophy is highly varied, ranging from early
childhood to later adulthood. The disease is characterized by muscle weakness and atrophy of the
muscles of the hip and shoulder areas (the limb girdles).
Facioscapulohumeral dystrophy (FSHD) typically appears before the age of 20, but can
appear later in adulthood or even in childhood in both males and females. FSHD affects the
muscles of the face, around the shoulder blades, and in the upper arms.
There is currently no cure for muscular dystrophy, but various treatments can help
manage symptoms and slow the progression of the disease. These may include physical therapy,
assistive devices, orthopedic surgery, and medication to manage muscle spasms and pain. In
some cases, genetic therapy and stem cell transplantation are also being explored as potential
treatment options.
It's important for individuals with muscular dystrophy to work closely with a healthcare
team that includes specialists in neurology, rehabilitation medicine, and orthopedics, to develop a
comprehensive care plan that meets their specific needs.
Bioengineering solutions for muscular dystrophy
Bioengineering solutions for muscular dystrophy aim to improve the lives of individuals
affected by the disease by addressing the underlying genetic mutations and muscle weakness.
Some of the approaches being explored include:
Gene therapy: This involves delivering a functional copy of the missing or mutated gene to
the affected muscle cells. The goal is to restore the production of the missing protein and
improve muscle function.
Stem cell therapy: This involves using stem cells to replace the damaged muscle cells and
promote repair and regeneration of the muscle tissue. Stem cells can be taken from the
patient's own body (autologous stem cells) or from a donor (allogenic stem cells).
Exoskeleton technology: This involves using wearable devices, such as robotic
exoskeletons, to support and enhance the movement of individuals with muscular dystrophy.
The devices use motors and sensors to mimic the movements of the wearer and help
improve mobility.
Tissue engineering: This involves using a combination of materials, such as scaffolds and
growth factors, to promote the growth and repair of muscle tissue. The goal is to create
functional muscle tissue that can replace the damaged tissue in individuals with muscular
dystrophy.
These approaches are still in the early stages of development, but hold promise for the
future treatment of muscular dystrophy. Clinical trials and further research are needed to
determine the safety and efficacy of these therapies.
6 / Whatsapp +91 7975054865
21BE45 Biology for Engineers, Common to All 4 th Semester Branches of VTU, Karnataka
Dr. Prasad Puthiyillam YouTube Channel: @ myintuition4865
3.3.5 Artificial Muscles
Artificial muscle refers to a type of technology that aims to mimic the properties and
functions of natural muscle. Artificial muscles can be made from various materials, including
shape memory alloys, electroactive polymers, and carbon nanotubes.
Shape Memory Alloys (SMAs) are materials with the ability to remember and recover
their original shape after being deformed. SMAs, like nickel-titanium (NiTi) alloys, are
commonly used in artificial muscle applications. When exposed to heat or an electric current,
SMAs undergo a phase transformation, enabling them to contract and generate force. This
property makes them suitable for mimicking muscle-like movements in devices such as
prosthetics, robotics, and actuators. The unique combination of shape memory and
superelasticity in SMAs provides excellent mechanical properties and durability for artificial
muscle applications.
Electroactive Polymers (EAPs) are a class of materials that exhibit significant changes in
shape or size when subjected to an electric field. These polymers, such as polypyrrole and
polyacrylonitrile, have the ability to undergo large deformation and respond quickly to electrical
stimulation. EAPs are particularly advantageous for artificial muscle applications due to their
lightweight nature, flexibility, and biocompatibility. They can be designed to contract or expand
in response to electrical signals, enabling precise control and mimicry of muscle-like
movements. EAPs have promising potential in areas such as soft robotics, haptic devices, and
biomedical applications.
Carbon Nanotubes (CNTs) are cylindrical structures composed of carbon atoms,
exhibiting exceptional mechanical, electrical, and thermal properties. CNTs possess high tensile
strength and are highly conductive, making them suitable for artificial muscle development. By
utilizing the electromechanical properties of CNTs, they can act as actuators that contract or
expand when stimulated by an electric current. CNT-based artificial muscles offer advantages
such as high power-to-weight ratio, fast response times, and potential scalability. Research is
underway to optimize CNT-based artificial muscles for applications in robotics, aerospace, and
microelectromechanical systems (MEMS).
Artificial muscles have a number of potential applications, including:
Robotics: Artificial muscles can be used to create more advanced and flexible robots that
can move and perform tasks more like humans.
Prosthetics: Artificial muscles can be used to create more advanced prosthetic limbs that are
more responsive and capable of performing a wider range of movements.
Biomedical devices: Artificial muscles can be used in various biomedical devices, such as
heart assist pumps and artificial hearts, to improve their performance and reliability.
Textile and clothing applications: Artificial muscles can be integrated into textiles and
clothing to create smart garments that can change shape and adjust to the wearer's
movements.
Artificial muscles have several advantages over traditional motors, including higher
power-to-weight ratios, faster response times, and greater flexibility. However, the technology is
still in the early stages of development and further research is needed to fully realize its potential
and overcome its limitations. https://youtu.be/FV5B653B2_k
7 / Whatsapp +91 7975054865
You might also like
- 3.3 Muscle System As ScaffoldDocument7 pages3.3 Muscle System As ScaffoldSantosh hiremathNo ratings yet
- AnaPhysio MSDocument5 pagesAnaPhysio MSJay Rodren IbitaNo ratings yet
- TE - Regeneration of Muscle by R Shweta and AryanDocument20 pagesTE - Regeneration of Muscle by R Shweta and AryanShweta RajanNo ratings yet
- Laprak MuscleDocument12 pagesLaprak MuscleRezky AmaliahNo ratings yet
- Advanced Models of Human Skeletal Muscle Differentiation - 2021 - Current OpiniDocument13 pagesAdvanced Models of Human Skeletal Muscle Differentiation - 2021 - Current OpinimetrorespoNo ratings yet
- Title: Structure & Function of Living CellsDocument10 pagesTitle: Structure & Function of Living CellsFatima Al SayyedNo ratings yet
- Skeletal MuscleDocument32 pagesSkeletal MuscleArfiNo ratings yet
- Muscular-System-Handouts - BiologyDocument7 pagesMuscular-System-Handouts - BiologyApril Joy T. GrijaldoNo ratings yet
- Unit Iv Soft Tissue Mechanics The Soft Tissues of The Body:: Kripa's Notes.Document14 pagesUnit Iv Soft Tissue Mechanics The Soft Tissues of The Body:: Kripa's Notes.Kripa NNo ratings yet
- Biol, J - Module 2Document6 pagesBiol, J - Module 2Jeric BiolNo ratings yet
- Bio 2100 Lab 3Document19 pagesBio 2100 Lab 3Kayseri PersaudNo ratings yet
- Kelompok 5.Document15 pagesKelompok 5.kadek sindyyNo ratings yet
- Biology Lect1. Type of Tissue DR - Aida AlobaidiDocument22 pagesBiology Lect1. Type of Tissue DR - Aida AlobaidiYaqeen AlaidyNo ratings yet
- Microscopic Anatomy of Skeletal MuscleDocument9 pagesMicroscopic Anatomy of Skeletal MuscleIan DonorNo ratings yet
- Exploring The World of Microscopic AnatomyDocument5 pagesExploring The World of Microscopic AnatomyMark Russel Sean LealNo ratings yet
- 2) SKELETAL MUSCLES A) Basic Skeletal Muscle PhysiologyDocument17 pages2) SKELETAL MUSCLES A) Basic Skeletal Muscle Physiologyfebruary141979No ratings yet
- Structure of Muscular SystemDocument10 pagesStructure of Muscular System32 Mhd RayisNo ratings yet
- StocumDocument5 pagesStocumLucía Carolina VidalNo ratings yet
- Application of Tissue EngineeringDocument3 pagesApplication of Tissue Engineeringtahomag44No ratings yet
- Histology Hand OutsDocument3 pagesHistology Hand OutsRemigio T. Arguilles Jr.No ratings yet
- Skeletal MuscularDocument14 pagesSkeletal Muscularapi-296073547No ratings yet
- Answer Sheet For ActivitiesDocument7 pagesAnswer Sheet For ActivitiesAlanah JaneNo ratings yet
- Tissue EngineeringDocument12 pagesTissue EngineeringDevanand GuptaNo ratings yet
- Exercise Physiology Lecture Note (Specialization)Document97 pagesExercise Physiology Lecture Note (Specialization)Shameem AliNo ratings yet
- 3 Types of Muscle TissueDocument11 pages3 Types of Muscle TissueJohnMichaelFabellaRuizNo ratings yet
- 3.4 - Connective Tissue - Medicine LibreTextsDocument13 pages3.4 - Connective Tissue - Medicine LibreTextsyigermalamanuel32No ratings yet
- AcknowledgmentsDocument5 pagesAcknowledgmentsjakjuNo ratings yet
- Extracellular Matrix - The Critical Contributor To Skeletal Muscle Regeneration-A Comprehensive Review (DIC-15-2023)Document15 pagesExtracellular Matrix - The Critical Contributor To Skeletal Muscle Regeneration-A Comprehensive Review (DIC-15-2023)Mario CorreaNo ratings yet
- Tissue Engineering: From Wikipedia, The Free EncyclopediaDocument12 pagesTissue Engineering: From Wikipedia, The Free EncyclopediaAnonymous ytZsBOVNo ratings yet
- Connective TissueDocument6 pagesConnective TissuecrtgyhujikNo ratings yet
- UNIT 3 (Muscular System)Document7 pagesUNIT 3 (Muscular System)Workinesh Kaynabo KambaloNo ratings yet
- Tissues 2Document48 pagesTissues 2chikwatumilessNo ratings yet
- Lec 6Document84 pagesLec 6Kazi Al MasumNo ratings yet
- Anatomy & Physiology The Muscular System Flashcards - QuizletDocument5 pagesAnatomy & Physiology The Muscular System Flashcards - Quizletmalenya1No ratings yet
- Anaphy ReportingDocument8 pagesAnaphy ReportingRushyl Angela FaeldanNo ratings yet
- Unit 5 - Muscle Tissue - Praktikum Struktur HewanDocument12 pagesUnit 5 - Muscle Tissue - Praktikum Struktur HewanSayNuruLNo ratings yet
- Advanced Anatomy - Myofascial MeridiansDocument37 pagesAdvanced Anatomy - Myofascial MeridiansBioTaoQi100% (2)
- Biology Contents - RPSC FSO by Food TecKnowDocument35 pagesBiology Contents - RPSC FSO by Food TecKnowRahul JainNo ratings yet
- Ns Elec 4 - Muscular SystemDocument4 pagesNs Elec 4 - Muscular SystemONGLENGCO WILSON JAKE V.No ratings yet
- Tissue Repair 2023PH17Document11 pagesTissue Repair 2023PH172023ph17No ratings yet
- Stem Cell Therapy For Rotator Cuff InjuriesDocument12 pagesStem Cell Therapy For Rotator Cuff InjuriesAthenaeum Scientific PublishersNo ratings yet
- Ns Elec 4 - Muscular SystemDocument5 pagesNs Elec 4 - Muscular SystemONGLENGCO WILSON JAKE V.No ratings yet
- Abella, Franz Karl - Zoology Reviewer Pt. 1Document4 pagesAbella, Franz Karl - Zoology Reviewer Pt. 1Franz AbellaNo ratings yet
- Muscular TissuDocument3 pagesMuscular Tissunikifarnaz8No ratings yet
- ANPH-M2-CU5. Muscular SystemDocument27 pagesANPH-M2-CU5. Muscular SystemMary Grace MapulaNo ratings yet
- Activity No.1 Muscular System 2nd Semester-2020-2021 1. What Is Muscular System?Document3 pagesActivity No.1 Muscular System 2nd Semester-2020-2021 1. What Is Muscular System?Leslie QuinonesNo ratings yet
- Muscular SystemDocument29 pagesMuscular SystemJoanna Alecxa PagunsanNo ratings yet
- Musculoskeletal SystemDocument54 pagesMusculoskeletal SystemYusuf HidayatNo ratings yet
- The Muscular TissueDocument23 pagesThe Muscular TissueMuhammad Salisu IliyasuNo ratings yet
- Bioengineering Solution For Muscle DytrophyDocument10 pagesBioengineering Solution For Muscle DytrophyAim HeadNo ratings yet
- Generation of HeatDocument2 pagesGeneration of HeatFani Try Utami BoedaxRed'waterzzNo ratings yet
- HKS123 Introduction To Sports Science Note-1Document32 pagesHKS123 Introduction To Sports Science Note-1ikponmwosavera1No ratings yet
- Pyogenic Discitis Power PointDocument121 pagesPyogenic Discitis Power Pointjav_luv37No ratings yet
- Presentation Fascial ManipulationDocument8 pagesPresentation Fascial ManipulationricardoNo ratings yet
- Team Pressure FinalDocument6 pagesTeam Pressure FinalsmurfseatmenNo ratings yet
- CEP HISTO - Lab Exercise 3 - Musculoskeletal TissuesDocument7 pagesCEP HISTO - Lab Exercise 3 - Musculoskeletal TissuesPia CincoNo ratings yet
- Human Anatomy and Physiology W3 - EmmanuelaDocument5 pagesHuman Anatomy and Physiology W3 - EmmanuelaRamizNo ratings yet
- BonesDocument6 pagesBones1NC21IS038 POLATHALA MOUNIKANo ratings yet
- Human Organ Systems and Bio Designs - 1: at Myintuition4865Document8 pagesHuman Organ Systems and Bio Designs - 1: at Myintuition48651NC21IS038 POLATHALA MOUNIKANo ratings yet
- HEARTDocument11 pagesHEART1NC21IS038 POLATHALA MOUNIKANo ratings yet
- KIDNYDocument7 pagesKIDNY1NC21IS038 POLATHALA MOUNIKANo ratings yet
- Medium Access Control Sub Layer ProtocolDocument8 pagesMedium Access Control Sub Layer Protocol1NC21IS038 POLATHALA MOUNIKANo ratings yet
- BonesDocument6 pagesBones1NC21IS038 POLATHALA MOUNIKANo ratings yet
- ProjectDocument6 pagesProject1NC21IS038 POLATHALA MOUNIKANo ratings yet
- ProjectDocument6 pagesProject1NC21IS038 POLATHALA MOUNIKANo ratings yet
- ProjectDocument6 pagesProject1NC21IS038 POLATHALA MOUNIKANo ratings yet
- ESA-ESTL-TM-0065 01 - New Cage Designs - Ball Riding CagesDocument44 pagesESA-ESTL-TM-0065 01 - New Cage Designs - Ball Riding CagesAnonymous lEX5U51wOANo ratings yet
- Four Different Levels of MeasurementDocument2 pagesFour Different Levels of MeasurementMaria Isabella RuaYanaNo ratings yet
- HTML-PS00001441 enDocument367 pagesHTML-PS00001441 enMarco Antonio Gutierrez Garduno100% (2)
- PTI Post-Tensioning Manual 6th EditionDocument362 pagesPTI Post-Tensioning Manual 6th Editionjackj_1820739838% (12)
- Plantech Industries Company Deck v1Document19 pagesPlantech Industries Company Deck v1Nemanja RadićNo ratings yet
- HRM 3703 Exam Prep ALL Chapters - CarolineDocument60 pagesHRM 3703 Exam Prep ALL Chapters - CarolineTessa Mkhomole100% (3)
- Multi PLC Workbench Scientech 2482Document6 pagesMulti PLC Workbench Scientech 2482Nikhil ShahNo ratings yet
- SAVA LEAFLET A4 EN TyresDocument2 pagesSAVA LEAFLET A4 EN TyresFilipposNo ratings yet
- LV Cable Catalogue (Copper)Document49 pagesLV Cable Catalogue (Copper)tees220510No ratings yet
- BTS412BDocument14 pagesBTS412Bstodzathegreat100% (1)
- Level 9.1: Instruction ManualDocument26 pagesLevel 9.1: Instruction ManualakshayuppalNo ratings yet
- ICON Tutorial 2017Document160 pagesICON Tutorial 2017zstos vmNo ratings yet
- Research 2 Week 1-2 LessonsDocument62 pagesResearch 2 Week 1-2 LessonsJenn Imperial100% (1)
- z80.Eu-Tools For Virus AnalysisDocument3 pagesz80.Eu-Tools For Virus AnalysisVIC RODNo ratings yet
- Torch (Machine Learning)Document4 pagesTorch (Machine Learning)levin696No ratings yet
- Termux Tutorial Complete Termux Commands - CodeDocument3 pagesTermux Tutorial Complete Termux Commands - CodeDavid Burkhouse100% (4)
- AUD O - Compilation Quiz (Reviewer)Document3 pagesAUD O - Compilation Quiz (Reviewer)Angela Miles DizonNo ratings yet
- Solution Manual For Statistical Methods 3rd Edition Rudolf Freund Donna Mohr William Wilson Full DownloadDocument6 pagesSolution Manual For Statistical Methods 3rd Edition Rudolf Freund Donna Mohr William Wilson Full Downloadandreasmithpitornwgbc100% (35)
- HDFC Bank: Loan Closure LffierDocument3 pagesHDFC Bank: Loan Closure LffierShoaib RajputNo ratings yet
- Grading Rubric For Commercials 1Document2 pagesGrading Rubric For Commercials 1Teacher MoNo ratings yet
- Upload A Document For Free Download Access.: Select Files From Your Computer or Choose Other Ways To Upload BelowDocument2 pagesUpload A Document For Free Download Access.: Select Files From Your Computer or Choose Other Ways To Upload BelowMasoud MonjeziNo ratings yet
- Lesson 2. Communication and GlobalizationDocument27 pagesLesson 2. Communication and GlobalizationJdee Dianne D. Suelto3210310No ratings yet
- Callyn Naidoo - Networks 511 AssigmentDocument8 pagesCallyn Naidoo - Networks 511 AssigmentDeep InsanityxNo ratings yet
- Brochure For Structure 2021Document2 pagesBrochure For Structure 2021Miguel WindsorNo ratings yet
- Computer Science Paper Scheme (Marks Distribution)Document4 pagesComputer Science Paper Scheme (Marks Distribution)Honey BunnyNo ratings yet
- SPE 11579 Improved Well Stimulation With Resin-Coated ProppantsDocument8 pagesSPE 11579 Improved Well Stimulation With Resin-Coated ProppantsbayuNo ratings yet
- COOLPLUS-Water Cooled Heat ExchangerDocument10 pagesCOOLPLUS-Water Cooled Heat ExchangerCOOLPLUS OIL COOLERNo ratings yet
- Array DSDocument10 pagesArray DSsriNo ratings yet
- Using Machine Learning Algorithms To Analyze Crime Data: March 2015Document13 pagesUsing Machine Learning Algorithms To Analyze Crime Data: March 2015pavithraNo ratings yet
- E 1211 - 97 - RteymtetukveDocument6 pagesE 1211 - 97 - RteymtetukveLENDNo ratings yet