Professional Documents
Culture Documents
Foreign Supplier Reevaluation Form
Uploaded by
amir Shehzad0 ratings0% found this document useful (0 votes)
63 views2 pagesCopyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
63 views2 pagesForeign Supplier Reevaluation Form
Uploaded by
amir ShehzadCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 2
Workaid G
IMPORTER REEVALUATION REAPPROVAL
NAME DATE DATE (IF APPLICABLE)
DISCONTINUED USE DATE
ADDRESS QI APPROVAL
(IF APPLICABLE)
FSVP Foreign Supplier Reevaluation Form Example*
Foreign Supplier Address
Foreign Supplier Name
(location)
Imported Food Product(s)
Description, including
Food Product(s) Imported
Important Food Safety
Characteristics
Type of Reevaluation** (i.e., regularly
If “For Cause,” Describe***
scheduled, “for cause”)
Reevaluation Considerations and Results
Other new
Changes to the Supplier’s New Other information
Procedures, Practices, Significant related to the
and Processes New Import New New Warning Compliance Action(s) Supplier’s Safety of the
(1.505(a)(1)(iii)(A)) Alerts Recalls Letters ((1.505)(a)(1)(iii)(B)) Corrective Actions food†
Changes to Food Safety Hazard(s) Changes to Description of
Controlled by Foreign Supplier††, ††† Foreign Supplier Control(s)†††
Justification for Changed
Current Verification Activity(ies) Changes to Verification New Verification Records (i.e.,
Verification Activity(ies) and
and Frequency††† Activity(ies) and Frequency††† audit summaries, test results)*
Frequency†††
© 2017 IIT IFSH A3-19
Appendix 3
FSVP Foreign Supplier Reevaluation Form Example* (continued)
Assessment of Results of Foreign Supplier
Reevaluation‡
[Note: If the reevaluation was performed by
another entity (other than the foreign supplier)
include entity’s name, address, email, and date of
reevaluation.]
Corrective Action(s) Taken as a Result of the
Reevaluation‡‡
*All supporting documentation should be appended to this form.
**Foreign supplier performance and food risk must be evaluated at least every 3 years or “for cause.”
***"For cause" may include any changes in the supplier’s procedures, processes, and practices related to the safety of the food; new information
about the supplier’s compliance with food safety standards (e.g., import alerts, recalls, FDA warning letters); responsiveness of the foreign
supplier in correcting food safety problems; new information on food testing results; new audit results relating to the safety of the food; or other
food safety considerations.
†Includes previous and recent experience with the supplier (e.g., rejected shipments, lab results, audit summaries, or other food safety
information you may have outside of the government oversight context).
††If someone other than the foreign supplier controls the hazard(s), document who is controlling the hazards and note if written assurances are
required.
†††When a Serious Adverse Health Consequences Or Death to Humans or Animals (SAHCODHA) hazard in a food will be controlled by the foreign
supplier, the default verification procedure is the performance of a properly conducted onsite audit of the foreign supplier before initially
importing the food and at least annually thereafter (21 CFR 1.506(d)(2)).
‡If another entity (other than the foreign supplier) performs the foreign supplier reevaluation, you may meet your reevaluation requirements by
having your QI review and assess the entity’s reevaluation. Your review/assessment of the reevaluation must include documentation that the
reevaluation was conducted by a QI.
‡‡You must document all reevaluations and corrective actions taken, if any are necessary.
IMPORTER APPROVAL APPROVAL DATE
A3-20 © 2017 IIT IFSH
You might also like
- The Sarbanes-Oxley Section 404 Implementation Toolkit: Practice Aids for Managers and AuditorsFrom EverandThe Sarbanes-Oxley Section 404 Implementation Toolkit: Practice Aids for Managers and AuditorsNo ratings yet
- Foreign Supplier Verification Activity (Ies) WorksheetDocument1 pageForeign Supplier Verification Activity (Ies) Worksheetamir ShehzadNo ratings yet
- Operational Profitability: Systematic Approaches for Continuous ImprovementFrom EverandOperational Profitability: Systematic Approaches for Continuous ImprovementNo ratings yet
- Foreign Supplier Evaluation FormDocument1 pageForeign Supplier Evaluation Formamir ShehzadNo ratings yet
- Schaum's Outline of Principles of Accounting I, Fifth EditionFrom EverandSchaum's Outline of Principles of Accounting I, Fifth EditionRating: 5 out of 5 stars5/5 (3)
- Production Process Change: Application FormDocument1 pageProduction Process Change: Application FormDurai NaiduNo ratings yet
- In ProcessDocument5 pagesIn Processvg_vvgNo ratings yet
- TJ PCP 2018 0774 1Document22 pagesTJ PCP 2018 0774 1Welinton Alexander Nivar RuizNo ratings yet
- Change Control: DR - K. Venkateswara Raju & Mr. K. T. Sunil KumarDocument27 pagesChange Control: DR - K. Venkateswara Raju & Mr. K. T. Sunil Kumarmeenu sruthi priyaNo ratings yet
- Change Control Report: X Pharmaceutical Manufacturing Company Quality Assurance Department Ref. SOP No.: Sop-XxxDocument7 pagesChange Control Report: X Pharmaceutical Manufacturing Company Quality Assurance Department Ref. SOP No.: Sop-Xxxrouss1906No ratings yet
- INSP - 002 - Second - Party - Inspection - Report Version 3.0Document5 pagesINSP - 002 - Second - Party - Inspection - Report Version 3.0Eduard GadzhievNo ratings yet
- HTG365. QAC. Inspection and Test Plan REV 2. 2022 06 24 (2) MRADocument10 pagesHTG365. QAC. Inspection and Test Plan REV 2. 2022 06 24 (2) MRAGreg Rabulan100% (1)
- Part Sample WarrantDocument1 pagePart Sample WarrantmageroteNo ratings yet
- Heromotors Self Assessment2Document7 pagesHeromotors Self Assessment2Rohit TripathiNo ratings yet
- BA Skill AssessmentDocument17 pagesBA Skill Assessmentsekhar chanduNo ratings yet
- 29.QAV 2-4 Report - 08030-PQ3F-F1-029 FormatDocument8 pages29.QAV 2-4 Report - 08030-PQ3F-F1-029 FormatRS MANIKANDANNo ratings yet
- Purchasing approval and vendor performance testingDocument28 pagesPurchasing approval and vendor performance testingLiew Chee KiongNo ratings yet
- Pipe Support Inspection and Test PlanDocument5 pagesPipe Support Inspection and Test PlanNos GoteNo ratings yet
- ISO 14001 Administrative Flowchart ExamplesDocument17 pagesISO 14001 Administrative Flowchart ExamplesPercy MphulanyaneNo ratings yet
- Itp For Site Preparation & Earth WorksDocument17 pagesItp For Site Preparation & Earth WorksDaniel Martinez100% (1)
- Bps Product Certification Scheme Surveillance Audit Form: Company ProfileDocument1 pageBps Product Certification Scheme Surveillance Audit Form: Company ProfileLenin Rey PolonNo ratings yet
- 菲律宾ps,ICC,coe和soc的申请要求Document8 pages菲律宾ps,ICC,coe和soc的申请要求张正No ratings yet
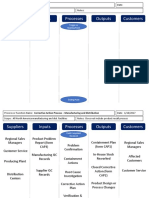
- SIPOC TemplateDocument3 pagesSIPOC TemplatesadaNo ratings yet
- Example Audit Program 2017Document24 pagesExample Audit Program 2017Hans DovonouNo ratings yet
- ICFR TemplateDocument6 pagesICFR Templatezeerakzahid100% (1)
- Supplier Approval, Monitoring & EvaluationDocument3 pagesSupplier Approval, Monitoring & EvaluationLuke BilisNo ratings yet
- 58 - Supplier Assessment Report - R12 - 06 08 2021Document10 pages58 - Supplier Assessment Report - R12 - 06 08 2021Sanjay KumarNo ratings yet
- 06_ExpenseReportToReimbursementDocument49 pages06_ExpenseReportToReimbursementsujit nayakNo ratings yet
- Product Quality Audit Check SheetDocument12 pagesProduct Quality Audit Check SheetscribdjnNo ratings yet
- Trai Qa R 45 Process Audit - FormatDocument5 pagesTrai Qa R 45 Process Audit - FormatRS MANIKANDANNo ratings yet
- ISI Proposal 11536Document5 pagesISI Proposal 11536Piyush GoyalNo ratings yet
- Sample PPAP Level-3Document36 pagesSample PPAP Level-3Mr. Mafia BhargavNo ratings yet
- L - FA - Niel T. - wk#06 - Contractors Monitoring & Measuring DeviceDocument1 pageL - FA - Niel T. - wk#06 - Contractors Monitoring & Measuring DeviceNIEL TANEDONo ratings yet
- Sop For Change ManagementDocument1 pageSop For Change ManagementGaurav Wadhwa0% (1)
- Share FileDocument3 pagesShare File张正No ratings yet
- Award of ContractDocument1 pageAward of ContractalliceyewNo ratings yet
- Management System Audit Report SummaryDocument9 pagesManagement System Audit Report Summarysajid waqasNo ratings yet
- ActionDocument4 pagesActionethan tardieuNo ratings yet
- Capitulo 9 Ingles Guia Ispe Volumen 5 1334122327Document10 pagesCapitulo 9 Ingles Guia Ispe Volumen 5 1334122327Daryl Barrios Lameda0% (1)
- 22 - Quality Equivalence Verification Report - Rev3Document3 pages22 - Quality Equivalence Verification Report - Rev3LokNo ratings yet
- CRF change request formDocument3 pagesCRF change request formswaran autoqaNo ratings yet
- Sub Contracting External ProductionDocument7 pagesSub Contracting External ProductionAnilNo ratings yet
- ITP Buckling Pin Relief Valves Inspection Test PlanDocument4 pagesITP Buckling Pin Relief Valves Inspection Test PlanjfdlksaNo ratings yet
- QAV-2 Step-3 Audit check sheet-PDFDocument5 pagesQAV-2 Step-3 Audit check sheet-PDFRajNo ratings yet
- Itp For Plumbing Amp Drainage SystemDocument98 pagesItp For Plumbing Amp Drainage Systemtristan guarinoNo ratings yet
- Food Hazard Analysis (HA) FormDocument1 pageFood Hazard Analysis (HA) Formamir ShehzadNo ratings yet
- Method of Statement For Internal External Oil Water Tanks Repairing and Coating SystemDocument19 pagesMethod of Statement For Internal External Oil Water Tanks Repairing and Coating SystemGaneshNo ratings yet
- Medical Device QMS - GMP System and AuditDocument27 pagesMedical Device QMS - GMP System and AuditShankar BNo ratings yet
- Main Inspection Test Plan SampleDocument2 pagesMain Inspection Test Plan SampleMuhd AfiqNo ratings yet
- Site Acceptance Testing (S.A.T) : Quality Control DepartmentDocument2 pagesSite Acceptance Testing (S.A.T) : Quality Control Departmentefmartin21No ratings yet
- Site Inspection ReportDocument44 pagesSite Inspection ReportOkan KalendarNo ratings yet
- HYG Part Approval FormsDocument24 pagesHYG Part Approval Formsmarcos coelhoNo ratings yet
- Inspection and Test PlanDocument10 pagesInspection and Test PlanixoteeNo ratings yet
- Quality Review Product NameDocument10 pagesQuality Review Product NameAndreas StathatosNo ratings yet
- CMS General AN Client 2 90 Annex SL - B57F49Document18 pagesCMS General AN Client 2 90 Annex SL - B57F49Keerti BonguNo ratings yet
- IncomingDocument8 pagesIncomingvg_vvgNo ratings yet
- 2 - 685 - PPAP PSW Submission+checklist - instructions+FAU F SPG 2430 EN 4Document8 pages2 - 685 - PPAP PSW Submission+checklist - instructions+FAU F SPG 2430 EN 4Helton MotaNo ratings yet
- Annex C - Change Request Form - 3 EditionDocument2 pagesAnnex C - Change Request Form - 3 EditionEliane CostaNo ratings yet
- BSD-GL-HAL-HMS-100 - (Terms & Definitions)Document42 pagesBSD-GL-HAL-HMS-100 - (Terms & Definitions)Eduard GadzhievNo ratings yet
- Only Upon GA Drawings Code 1/2 Approval From DPJV.: by Sekar - Rajesh at 6:43 PM, Aug 25, 2015Document4 pagesOnly Upon GA Drawings Code 1/2 Approval From DPJV.: by Sekar - Rajesh at 6:43 PM, Aug 25, 2015Rahmat BasukiNo ratings yet
- Form 3 BDocument3 pagesForm 3 Bamir ShehzadNo ratings yet
- FSVP Awareness Module Foreign Suppliers - v1.0 Participant Manual 2017 Handout EnglishDocument34 pagesFSVP Awareness Module Foreign Suppliers - v1.0 Participant Manual 2017 Handout Englishamir ShehzadNo ratings yet
- Determining The FSVP ImporterDocument1 pageDetermining The FSVP Importeramir ShehzadNo ratings yet
- Biosafety FH Guidance Listeria-Monoc Profel enDocument59 pagesBiosafety FH Guidance Listeria-Monoc Profel enamir ShehzadNo ratings yet
- Minimum Growth Temperatures for Foodborne PathogensDocument10 pagesMinimum Growth Temperatures for Foodborne PathogensGiancarloNo ratings yet
- 2 - CookingTemperature LogDocument2 pages2 - CookingTemperature Logamir ShehzadNo ratings yet
- Zone 1 Sampling For Spp. in Fresh Produce Operations:: ListeriaDocument10 pagesZone 1 Sampling For Spp. in Fresh Produce Operations:: Listeriaamir ShehzadNo ratings yet
- HACCP Supporting DocumentsDocument221 pagesHACCP Supporting Documentsamir ShehzadNo ratings yet
- 4 - Thermometer Caliberation LogDocument2 pages4 - Thermometer Caliberation Logamir ShehzadNo ratings yet
- Ha CCP Master SheetDocument1 pageHa CCP Master Sheetamir ShehzadNo ratings yet
- 5 - Receiving LogDocument2 pages5 - Receiving Logamir ShehzadNo ratings yet
- 1 Paper 691 MOMENA I WELFARE IMPLICATIONS OF ALTERNATIVE POLICY OPTIONS Analysis of Wheat Market in Pakistan 1Document25 pages1 Paper 691 MOMENA I WELFARE IMPLICATIONS OF ALTERNATIVE POLICY OPTIONS Analysis of Wheat Market in Pakistan 1amir ShehzadNo ratings yet
- Effect of Different Gums On The Quality and ShelfDocument2 pagesEffect of Different Gums On The Quality and Shelfamir ShehzadNo ratings yet
- Finding Relevant Supporting Documentation MaterialsDocument4 pagesFinding Relevant Supporting Documentation Materialsamir ShehzadNo ratings yet
- Bread: Dough Mixing and Testing OperationsDocument10 pagesBread: Dough Mixing and Testing Operationsamir ShehzadNo ratings yet
- Accepted Manuscript: Food ChemistryDocument30 pagesAccepted Manuscript: Food Chemistryamir ShehzadNo ratings yet
- Recent Developments in Dough-Based Bakery Products: A Mini ReviewDocument6 pagesRecent Developments in Dough-Based Bakery Products: A Mini Reviewamir ShehzadNo ratings yet
- Comparative Advantage of Wheat Crop in PakistanDocument11 pagesComparative Advantage of Wheat Crop in Pakistanamir ShehzadNo ratings yet
- Chemical Cooking and Sensory Characteristics of BuDocument9 pagesChemical Cooking and Sensory Characteristics of Buamir ShehzadNo ratings yet
- Estelle R 2006Document4 pagesEstelle R 2006amir ShehzadNo ratings yet
- Wheat Bran-Composition and Nutritional Quality: A Review: Research ArticleDocument7 pagesWheat Bran-Composition and Nutritional Quality: A Review: Research Articleamir ShehzadNo ratings yet
- The Contribution of Wheat To Human Diet and Health: ReviewDocument25 pagesThe Contribution of Wheat To Human Diet and Health: Reviewamir ShehzadNo ratings yet
- Naheed Zia Khan, Munir Ahmed and Asia RasheedDocument29 pagesNaheed Zia Khan, Munir Ahmed and Asia Rasheedtassawar1816No ratings yet
- Claus 2008Document9 pagesClaus 2008amir ShehzadNo ratings yet
- Gomal-8, A High Yielding, Disease Tolerant Wheat Variety For Irrigated AreasDocument5 pagesGomal-8, A High Yielding, Disease Tolerant Wheat Variety For Irrigated Areasamir ShehzadNo ratings yet
- Study of Most Prevalent Wheat Seed-Borne Mycoflora and Its Effect On Seed Nutritional ValueDocument10 pagesStudy of Most Prevalent Wheat Seed-Borne Mycoflora and Its Effect On Seed Nutritional Valueamir ShehzadNo ratings yet
- 7 Wheat Proteins: Structure and Functionality in Milling and HreadmakingDocument2 pages7 Wheat Proteins: Structure and Functionality in Milling and Hreadmakingamir ShehzadNo ratings yet
- New Wheat Variety "Fareed-06" For Irrigated Areas of Punjab, PakistanDocument13 pagesNew Wheat Variety "Fareed-06" For Irrigated Areas of Punjab, Pakistanamir ShehzadNo ratings yet
- Performance of Some Improved Bread Wheat Varieties Grown in Khyber Pakhtunkhwa, PakistanDocument3 pagesPerformance of Some Improved Bread Wheat Varieties Grown in Khyber Pakhtunkhwa, Pakistanamir ShehzadNo ratings yet
- Chemical Composition and Functional Properties of Wheat Bread Containing Wheat and Legumes BranDocument6 pagesChemical Composition and Functional Properties of Wheat Bread Containing Wheat and Legumes BranAdoptedchildNo ratings yet
- Mahnia Wala Chak No 190 JB Post Office Khas Tehsil Chiniot Distt Muhammad Saleem Raza ShahDocument4 pagesMahnia Wala Chak No 190 JB Post Office Khas Tehsil Chiniot Distt Muhammad Saleem Raza ShahMUHAMMAD SALEEM RAZANo ratings yet
- AnalysisOfSection65 BDocument15 pagesAnalysisOfSection65 Bsiva KNo ratings yet
- Fifth Semester Commerce Corporate Accounting (CBCS - 2017 Onwards)Document12 pagesFifth Semester Commerce Corporate Accounting (CBCS - 2017 Onwards)VELAVAN ARUNADEVINo ratings yet
- Republic vs. FelicianoDocument10 pagesRepublic vs. Felicianoflordelei hocateNo ratings yet
- (O&M ) Satish Kumar Vs Laxmi Narain On 22 December, 2015Document17 pages(O&M ) Satish Kumar Vs Laxmi Narain On 22 December, 2015Nishant hasijaNo ratings yet
- Ontario v. Closeout Furniture - ComplaintDocument30 pagesOntario v. Closeout Furniture - ComplaintSarah BursteinNo ratings yet
- 0051 001Document4 pages0051 001Raheem KassamNo ratings yet
- Common Land and Common Property Resources: Issues of Equity in Rural Madhya Pradesh (New Delhi: Sage, 2002), P. 204Document14 pagesCommon Land and Common Property Resources: Issues of Equity in Rural Madhya Pradesh (New Delhi: Sage, 2002), P. 204Akif AbidiNo ratings yet
- Heirs of Malabanan vs. RepublicDocument12 pagesHeirs of Malabanan vs. RepublicMitch TinioNo ratings yet
- Prison Construction Draft BillDocument29 pagesPrison Construction Draft BillMike CasonNo ratings yet
- Budget Fy21Document138 pagesBudget Fy21ForkLogNo ratings yet
- Ansi Saia A92 5 2006 R2014 Boom Supported Elevating Work PlatformsDocument62 pagesAnsi Saia A92 5 2006 R2014 Boom Supported Elevating Work PlatformsLuis Carcamo Martinez100% (1)
- Solution. Investment 1: Simple Interest, With Annual Rate RDocument11 pagesSolution. Investment 1: Simple Interest, With Annual Rate RLucky Gemina67% (3)
- An Allonge Is Not An AssignmentDocument3 pagesAn Allonge Is Not An Assignmentdbush2778No ratings yet
- Uy Tuazon v. World WiserDocument2 pagesUy Tuazon v. World WiserNeil Aubrey GamidoNo ratings yet
- Internal Audit S Role in EsgDocument4 pagesInternal Audit S Role in EsgDumNo ratings yet
- Was Ewig Liegt SpielerleitfadenPDF LZ MetaDocument12 pagesWas Ewig Liegt SpielerleitfadenPDF LZ MetaSachmeth94No ratings yet
- FINAL CivRev Property TSN 1Document187 pagesFINAL CivRev Property TSN 1Patrick CanamaNo ratings yet
- Anju Sharma U1 283 Grange Road, Ormond VIC 3204Document1 pageAnju Sharma U1 283 Grange Road, Ormond VIC 3204Aksh GillNo ratings yet
- Rules for Suspensive and Resolutory Conditions in the PhilippinesDocument6 pagesRules for Suspensive and Resolutory Conditions in the PhilippinesmacrosalNo ratings yet
- Civil Service Form GuideDocument3 pagesCivil Service Form GuideStephanie PayumoNo ratings yet
- Full Test Bank For CCH Federal Taxation Comprehensive Topics 2014 Harmelink 080802972X PDF Docx Full Chapter ChapterDocument36 pagesFull Test Bank For CCH Federal Taxation Comprehensive Topics 2014 Harmelink 080802972X PDF Docx Full Chapter Chapterfuze.riddle.ghik9100% (11)
- Human Rights Group Assignment Analyzes ICC Prosecution ProcessDocument4 pagesHuman Rights Group Assignment Analyzes ICC Prosecution ProcessFrank Lloyd CadornaNo ratings yet
- Benjamin FranklinDocument16 pagesBenjamin FranklinBiblioteca ContaderoNo ratings yet
- Aznar v. GarciaDocument2 pagesAznar v. GarciaReigh Harvy CantaNo ratings yet
- BinderDocument3 pagesBinderBK AcharyaNo ratings yet
- Judicial Appointments in PakistanDocument9 pagesJudicial Appointments in PakistanFURQAN AHMEDNo ratings yet
- 092 Nha Vs EvangelistaDocument4 pages092 Nha Vs EvangelistaUE LawNo ratings yet
- 1 Veloso V DOLEDocument3 pages1 Veloso V DOLEDominique LarinNo ratings yet
- Maths 2 Answer Sheet PDFDocument1 pageMaths 2 Answer Sheet PDFvinitha2675No ratings yet