Professional Documents
Culture Documents
Error Analysis Notes
Uploaded by
Naysa RenjuCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Error Analysis Notes
Uploaded by
Naysa RenjuCopyright:
Available Formats
Name: Error Analysis
Percent Difference
When asked to compare two values, one helpful technique is to use Percent Difference.
There are three times when you might use this technique.
1. Experimental to Theoretical (predicted) Value
Theoretical − Experimental
% difference = 100%
Theoretical
2. Experimental to Better Experimental Value (one that is more reliable)
Experimental − Better
% difference = 100%
Better
3. Two Experimental Values
Value1 − Value2
% difference = 100%
SmallerValue
Percent Differences have one significant figure, unless the percent difference is greater
than 10% (then use 2 significant figures).
Precision Measure
Precision Measure is how precise a measurement can be made with the measuring device. The
precision measure is usually one-half of the smallest interval marked on the instrument (or
which can be determined without estimation). Meter stick = ±0.05cm
Absolute Error
The absolute error is an estimation of the actual value. Usually, this is the value of a data
with the precision measure attached.
40.3 0.1 cm
% Error
The % error is the absolute error in percentage form. It is used only for calculations.
0.1
40.3 0.1=40.3 100%
40.3 Please note that the error has 2 sig. fig.
= 40.3 0.25%
Answers are always given in Absolute Error form.
Rules for dealing with errors
Adding or Subtracting Quantities
- the absolute errors are added together
(42.1 0.1) + (41.6 0.1) = 83.7 0.2
(42.1 0.1) − (41.6 0.1) = 0.5 0.2
Multiplying or Dividing Quantities
- the absolute errors are converted to %errors, and the %errors are added together
52.01 0.05 52.01 0.096%
=
28.06 0.05 28.06 0.18%
.28 x1.8535
= 1.8535 0.28% The % error is then converted to an absolute error.
100
= 1.8535 0.0052
= 1.854 0.005
Multiplying Quantity by a Constant
- the absolute errors are multiplied by the constant
2 (42.1 0.1) = 264.52 0.63
= 264.5 0.6
Raising a Quantity to a Power
- multiply the %error by the power
(4.3 0.1) 2 = (4.3 2.3%) 2
= 18.49 4.6%
= 18.49 0.85
= 18.5 0.8
Experimental Errors for Discussion Purposes
Experiments usually have associated errors. There are a couple of kinds of errors that can
occur in experiments that must be reported at a high-school level.
Systematic Errors
Errors that are consistently slightly greater (or lower) than the expected valued are
systematic errors. These errors are regular and predictable. Usually the results are
changed based on a constant amount, or a constant ratio and the predictable nature of the
change is an indicator of a systematic error.
Random Errors
When data vary unpredictably from expected data, we say that the errors are random. Small
amounts of friction on an air table may cause data to have random errors associated. These
random errors are usually because the experiment cannot control all the variables that may
affect the results.
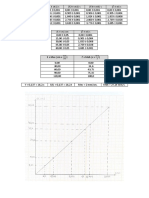
Graph 1.1 The average velocity of a ball rolling
down an inclined plane vs. Time
80
0.025sec
x = (0.50block )( )
block
x = 0.0125s
• 2.0cms −1
60
y = (0.50block )( )
block
y = 1.0cm s −1
•
V • (68.0 1.0cms −1 ) − (4.00 1.0cms −1 )
Slope =
40 (0.715 0.0125s ) − (0.050 0.0125s)
(cm•s-1)
• 64.0 2.0cms −1
=
0.665 0.025s
• 64.0 3.1%cms −1
=
20 0.665 3.8% s
= 96.24 6.9% cms −1
• = 96.24 6.64 cms −1
• = 96.2 6.6cms −1
0
0.10 0.20 0.30 0.40 0.50 0.60 0.70 0.80
t
(s)
You might also like
- Math Practice Simplified: Decimals & Percents (Book H): Practicing the Concepts of Decimals and PercentagesFrom EverandMath Practice Simplified: Decimals & Percents (Book H): Practicing the Concepts of Decimals and PercentagesRating: 5 out of 5 stars5/5 (3)
- Airs-Lms - Math-10 - q3 - Week 3-4 Module 3 Rhonavi MasangkayDocument19 pagesAirs-Lms - Math-10 - q3 - Week 3-4 Module 3 Rhonavi MasangkayRamil J. Merculio100% (1)
- Method of Reporting Screen Analysis & Factors Affecting Screening EfficiencyDocument18 pagesMethod of Reporting Screen Analysis & Factors Affecting Screening EfficiencyAbhijit NathNo ratings yet
- PROBLEM SET-4 Continuous Probability - SolutionsDocument8 pagesPROBLEM SET-4 Continuous Probability - Solutionsmaxentiuss71% (7)
- Instructor's Manual to Accompany CALCULUS WITH ANALYTIC GEOMETRYFrom EverandInstructor's Manual to Accompany CALCULUS WITH ANALYTIC GEOMETRYNo ratings yet
- Pebc CompilationDocument14 pagesPebc CompilationAarti AroraNo ratings yet
- 2010 HSC Exam PhysicsDocument42 pages2010 HSC Exam PhysicsVictor345No ratings yet
- Best Way To LearnDocument3 pagesBest Way To LearnRAMIZKHAN124No ratings yet
- Oracle Unified Method (OUM) White Paper - Oracle's Full Lifecycle Method For Deploying Oracle-Based Business Solutions - GeneralDocument17 pagesOracle Unified Method (OUM) White Paper - Oracle's Full Lifecycle Method For Deploying Oracle-Based Business Solutions - GeneralAndreea Mirosnicencu100% (1)
- Heat and Mass Transfer Distillation ProblemsDocument10 pagesHeat and Mass Transfer Distillation ProblemsMJNo ratings yet
- Digital Electronics For Engineering and Diploma CoursesFrom EverandDigital Electronics For Engineering and Diploma CoursesNo ratings yet
- ClientsDocument7 pagesClientsLiane PanahacNo ratings yet
- Masterbatch Buyers Guide PDFDocument8 pagesMasterbatch Buyers Guide PDFgurver55No ratings yet
- STA60104 TutorialsDocument123 pagesSTA60104 TutorialsSabrina NgoNo ratings yet
- 3 8 A TheEmpiricalRuleDocument9 pages3 8 A TheEmpiricalRuleGage HolmesNo ratings yet
- Chapter 1 Lesson 4 Variance and Standard DeviationDocument14 pagesChapter 1 Lesson 4 Variance and Standard DeviationAndrenz EGNo ratings yet
- CM1A - Mock Exam Sept 23 SolutionDocument8 pagesCM1A - Mock Exam Sept 23 SolutionKrishna JhanwarNo ratings yet
- Finals Assessment 2Document4 pagesFinals Assessment 2ShieNo ratings yet
- Least Square FilterDocument5 pagesLeast Square Filterduc2711No ratings yet
- Column Details Bill WiseDocument3 pagesColumn Details Bill WiseIrfan HabeebNo ratings yet
- Chemical Kinetics Simulator: An Interactive Graphical ApproachDocument8 pagesChemical Kinetics Simulator: An Interactive Graphical Approachnurul ismiNo ratings yet
- Method 1: Median 95% CI Simple Method PerDocument13 pagesMethod 1: Median 95% CI Simple Method PersoasabNo ratings yet
- Decision ScienceDocument8 pagesDecision ScienceHimanshi YadavNo ratings yet
- Nguyễn Luận Công Bằng ITITIU20163 HW11 12Document6 pagesNguyễn Luận Công Bằng ITITIU20163 HW11 12Tú NgọcNo ratings yet
- Employee Job Satisfaction Analysis Before and After TrainingDocument9 pagesEmployee Job Satisfaction Analysis Before and After TrainingShehbazShoukatNo ratings yet
- Solution MatlabDocument46 pagesSolution MatlabFreddy Mendoza CoronelNo ratings yet
- Analyzing currency portfolio risk and maximum expected lossesDocument5 pagesAnalyzing currency portfolio risk and maximum expected lossesRidwan IslamNo ratings yet
- Component-A (MVC)Document16 pagesComponent-A (MVC)westewrNo ratings yet
- Assgn Numec Individu KamilDocument10 pagesAssgn Numec Individu KamilKamil BudimanNo ratings yet
- Lecture 7B-Tut T8 Example Lanes Approach For COG OptimisationDocument24 pagesLecture 7B-Tut T8 Example Lanes Approach For COG OptimisationElijah MuntembaNo ratings yet
- Summarizing Data - Statistical HydrologyDocument6 pagesSummarizing Data - Statistical HydrologyJavier Avila100% (1)
- Chem LabDocument16 pagesChem LabNickstar592No ratings yet
- Generating Random Numbers Using Various AlgorithmsDocument9 pagesGenerating Random Numbers Using Various AlgorithmsElviraNo ratings yet
- ESPECTRO T Vs C - RADocument40 pagesESPECTRO T Vs C - RAWilmer PradoNo ratings yet
- Abnormal Lab Values and Diagnostic Tests ResultsDocument9 pagesAbnormal Lab Values and Diagnostic Tests Resultsefka anindiaNo ratings yet
- 111-2 Data Analysis Assignment: I-cos2θ圖Document2 pages111-2 Data Analysis Assignment: I-cos2θ圖陳泓睿No ratings yet
- ConfidenceIntervals MSS Supp VideoDocument23 pagesConfidenceIntervals MSS Supp VideoannaNo ratings yet
- AttentionNumerical SolvedDocument15 pagesAttentionNumerical SolvedVishal KumarNo ratings yet
- Lista - Atividades - 1 4 5 12Document8 pagesLista - Atividades - 1 4 5 12Brenda CarneiroNo ratings yet
- Ecart TypeDocument3 pagesEcart TypeJihane AlouiNo ratings yet
- Regression Analysis Explains Relationship Between Speed & AccidentsDocument9 pagesRegression Analysis Explains Relationship Between Speed & AccidentsSneha BhaipNo ratings yet
- ecart typeDocument3 pagesecart typeChoaib NouhyNo ratings yet
- σs) cm σt1) s σt2) s σt3) s σt) sDocument1 pageσs) cm σt1) s σt2) s σt3) s σt) saf cNo ratings yet
- Test 1 MAc2022Document2 pagesTest 1 MAc2022Aqim AzamNo ratings yet
- Valencia Vs LevanteDocument2 pagesValencia Vs LevanteDick De la VegaNo ratings yet
- Lab Report 03 - Edm CalibrationDocument2 pagesLab Report 03 - Edm CalibrationShivam ShuklaNo ratings yet
- Metode Pendekatan Berurutan:: DiketahuiDocument4 pagesMetode Pendekatan Berurutan:: DiketahuiDinda Sari BintangNo ratings yet
- Lab 2 DatosDocument3 pagesLab 2 DatosMaria Del Pilar Viveros BenavidesNo ratings yet
- Nama: Fransiskus Giling NIM: 2010212397: Metode Pendekatan BerurutanDocument4 pagesNama: Fransiskus Giling NIM: 2010212397: Metode Pendekatan BerurutanDinda Sari BintangNo ratings yet
- Eenvoudige Lineêre Regressie Simple Linear RegressionDocument8 pagesEenvoudige Lineêre Regressie Simple Linear RegressionGregorNo ratings yet
- Opration Management TAMDocument5 pagesOpration Management TAMHK 'sNo ratings yet
- Equilibrium and Strength Lab ResultsDocument8 pagesEquilibrium and Strength Lab ResultsJackson WangNo ratings yet
- Tau ThomsonDocument2 pagesTau ThomsonCiobanu MihaiNo ratings yet
- A critical analysis of statistical methods in ISO 13528 for proficiency testing schemesDocument22 pagesA critical analysis of statistical methods in ISO 13528 for proficiency testing schemesAnonymous fdZ815WNo ratings yet
- Melde 2Document25 pagesMelde 2angelica.nilova2710No ratings yet
- Graphic IV.1 Related Between Strain and Stress Cylinder: 100 σ = 86,624 kg/cm σ = 84 kg/cmDocument10 pagesGraphic IV.1 Related Between Strain and Stress Cylinder: 100 σ = 86,624 kg/cm σ = 84 kg/cmkemalNo ratings yet
- Teknik SimulasiDocument5 pagesTeknik SimulasiBee BaiiNo ratings yet
- Modelo 1-2-3Document2 pagesModelo 1-2-3Daniel ZambranoNo ratings yet
- DOM 105 - 1 (Skew and Kurt)Document5 pagesDOM 105 - 1 (Skew and Kurt)Vanisha GuptaNo ratings yet
- BiostatDocument7 pagesBiostatyoan yulista hartivaNo ratings yet
- Juan Pablo Quiñones Quiz 3 CORTEDocument6 pagesJuan Pablo Quiñones Quiz 3 CORTEEdwinNo ratings yet
- Quantitative Group AssigmentDocument10 pagesQuantitative Group AssigmentAbrahamNo ratings yet
- Effect of Temperature on Benzoquinone Concentration Over TimeDocument2 pagesEffect of Temperature on Benzoquinone Concentration Over TimeGavanDusbabekNo ratings yet
- This Is Only For Practice and Will Not Be GradedDocument5 pagesThis Is Only For Practice and Will Not Be GradedVikash KumarNo ratings yet
- Tutorial 13 Confidence Interval (Proportion) - SOLUTIONSDocument5 pagesTutorial 13 Confidence Interval (Proportion) - SOLUTIONSTan Li XuanNo ratings yet
- Waktu (menit) Ekstrak Rafinat x x* Δx Δx Log Mean Driving Force Mass Transfer CoefficientDocument1 pageWaktu (menit) Ekstrak Rafinat x x* Δx Δx Log Mean Driving Force Mass Transfer CoefficientMella Frandista KNo ratings yet
- Experiment-2: Shear Centre of Open SectionsDocument7 pagesExperiment-2: Shear Centre of Open SectionsRahul RoyNo ratings yet
- 5.a Personal Diet Consultant For Healthy MealDocument5 pages5.a Personal Diet Consultant For Healthy MealKishore SahaNo ratings yet
- 3.1 C 4.5 Algorithm-19Document10 pages3.1 C 4.5 Algorithm-19nayan jainNo ratings yet
- Service Positioning and DesignDocument3 pagesService Positioning and DesignSaurabh SinhaNo ratings yet
- L Williams ResumeDocument2 pagesL Williams Resumeapi-555629186No ratings yet
- TILE FIXING GUIDEDocument1 pageTILE FIXING GUIDEStavros ApostolidisNo ratings yet
- 1 PBDocument11 pages1 PBAnggita Wulan RezkyanaNo ratings yet
- Load Frequency Control of Hydro and Nuclear Power System by PI & GA ControllerDocument6 pagesLoad Frequency Control of Hydro and Nuclear Power System by PI & GA Controllerijsret100% (1)
- Android TabletsDocument2 pagesAndroid TabletsMarcus McElhaneyNo ratings yet
- Inertial Reference Frames: Example 1Document2 pagesInertial Reference Frames: Example 1abhishek murarkaNo ratings yet
- Barcelona Smart City TourDocument44 pagesBarcelona Smart City TourPepe JeansNo ratings yet
- U-KLEEN Moly Graph MsdsDocument2 pagesU-KLEEN Moly Graph MsdsShivanand MalachapureNo ratings yet
- Barelwiyah, Barelvi Chapter 1 (Part 2 of 5)Document31 pagesBarelwiyah, Barelvi Chapter 1 (Part 2 of 5)Dawah ChannelNo ratings yet
- AIOUDocument2 pagesAIOUHoorabwaseemNo ratings yet
- Marulaberry Kicad EbookDocument23 pagesMarulaberry Kicad EbookPhan HaNo ratings yet
- GD&T WIZ Tutor Covers The Vast Breadth of Geometric Dimensioning and Tolerancing Without Compromising On The Depth. The Topics Covered AreDocument1 pageGD&T WIZ Tutor Covers The Vast Breadth of Geometric Dimensioning and Tolerancing Without Compromising On The Depth. The Topics Covered AreVinay ManjuNo ratings yet
- 41 PDFsam Redis CookbookDocument5 pages41 PDFsam Redis CookbookHữu Hưởng NguyễnNo ratings yet
- Vincent Ira B. Perez: Barangay Gulod, Calatagan, BatangasDocument3 pagesVincent Ira B. Perez: Barangay Gulod, Calatagan, BatangasJohn Ramsel Boter IINo ratings yet
- Edmonson - Pageantry Overture - AnalysisDocument3 pagesEdmonson - Pageantry Overture - Analysisapi-426112870No ratings yet
- Friction, Gravity and Energy TransformationsDocument12 pagesFriction, Gravity and Energy TransformationsDaiserie LlanezaNo ratings yet
- ProjectDocument86 pagesProjectrajuNo ratings yet
- True False Survey FinalDocument2 pagesTrue False Survey Finalwayan_agustianaNo ratings yet
- United Airlines Case Study: Using Marketing to Address External ChallengesDocument4 pagesUnited Airlines Case Study: Using Marketing to Address External ChallengesSakshiGuptaNo ratings yet
- Mechanical Engineer with Experience in Heavy Mining Machinery MaintenanceDocument1 pageMechanical Engineer with Experience in Heavy Mining Machinery MaintenanceCertified Rabbits LoverNo ratings yet