Professional Documents
Culture Documents
nn1c10602 Si 001
Uploaded by
Yassine MOUHIB0 ratings0% found this document useful (0 votes)
9 views24 pagesOriginal Title
nn1c10602_si_001
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
9 views24 pagesnn1c10602 Si 001
Uploaded by
Yassine MOUHIBCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 24
Supporting Information
Molecular Mechanisms of Early Flowering in Tomato
Induced by Manganese Ferrite (MnFe2O4) Nanomaterials
Le Yueab, Yan Fengab, Chuanxin Mac, Chuanxi Wangab, Feiran Chenab, Xuesong
Caoab, Jing Wangab, Jason C. Whited, Zhenyu Wang*ab, Baoshan Xinge
a Institute of Environmental Processes and Pollution Control and School of Environment and
Civil Engineering, Jiangnan University, Wuxi 214122, China.
b Jiangsu Engineering Laboratory for Biomass Energy and Carbon Reduction Technology,
Wuxi 214122, China.
c Key Laboratory for City Cluster Environmental Safety and Green Development of the Ministry
of Education, Institute of Environmental and Ecological Engineering, Guangdong University
of Technology, Guangzhou 510006, China.
d The Connecticut Agricultural Experiment Station, New Haven, Connecticut 06504,
United States
e Stockbridge School of Agriculture, University of Massachusetts, Amherst, Massachusetts
01003, United States.
*Corresponding author:
Tel.: +86 0510 85911123; Fax: +86 0510 85197773
E-mail address: wang0628@jiangnan.edu.cn (Dr. Zhenyu Wang)
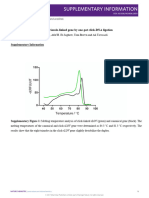
Text. S1 Optimum concentration selected to explore the promoting mechanisms of
tomato flowering
Fig. S4 shows the growth of tomato plants after spraying MnFe2O4 NMs at different
concentrations and different times. MnFe2O4 NMs significantly promoted flowering
time as the dose increased (Fig. S4a). After foliar spraying 10 and 50 mg L-1 MnFe2O4
NMs, the flower formation time was significantly reduced by 13 days and 15 days as
compared to control, respectively. At harvest, the fruit number of all tomato plants upon
1, 10 and 50 mg L-1 MnFe2O4 NMs treatment was significantly increased compared of
the control (Fig. S4b), and the sugar content in the fruits was 1.4, 2.0 and 1.2 times that
of the control, respectively (Fig. S4c). Therefore, 10 mg L-1 was chosen as the
concentration for subsequent experiments due to the best growth effects. Also, the net
photosynthetic rate of tomato leaves after 4 and 7 times of consecutive applications at
different concentrations was analyzed (Fig. S4d). After spraying 4 times, the net
photosynthetic rate of all treatments increased significantly, and the effects of 10 mg
L-1 and 50 mg L-1 were the greatest. However, only 1 mg L-1 had the better net
photosynthetic rate than control after 7 times of spraying. This might be because that
the increased continuous foliar spraying caused stomatal blockage. Fig. S4e further
verified that the stomatal conductance of tomato leaves increased significantly by 26.6%
after spraying 1 time but decreased significantly by 38.9% after spraying 7 times. In
addition, the sucrose content in leaves increased by 7.5% and 8.8% after spraying 10
mg L-1 MnFe2O4 NMs for 1 and 4 times, respectively (Fig. S4f). Therefore, 10 mg L-1
MnFe2O4 NMs spraying for 4 times was selected for further investigation.
Text. S2 HPLC-MS/MS operating parameters used in this study
After the plant sample extraction, 10 μL samples were injected onto a Acquity UPLC
HSS T3 (2.1×100 mm, 1.8μm) using an 18-min linear gradient at a flow rate of 0.35
mL/min. The eluents in negative/positive mode were eluent A (0.1% v/v formic acid in
water) and eluent B (0.1% formic acid in acetonitrile). The solvent gradient was set as
follows: 5% B, 0 min; 5% B, 1.5 min; 100% B, 14.0 min; 100% B, 15.5 min; 5% B, 16
min; 5% B, 18 min. Nitrogen was used as sheath gas (35 L / min) and aux gas (15 L /
min). The spray voltage was set as 3 kV (-3 kV for negative mode and 3 kV for positive
mode) and capillary temperature was 320 ℃. The resolution of full MS was set as 70000
and scan range was acquired between 70 to 1050 m/z in full MS-dd MS2 mode. The
following dd-MS2 product ion spectra were collected at resolution of 17500, isolation
window of 1.5 m/z, and collision energy of nce: 20, 40, 60. Compounds were identified
based on their retention time and mass spectrum values in the mzCloud and mzVault
library.
Fig. S1. Zeta potential, hydrodynamic diameter, and TEM image (a), size distribution
(b), and VSM (d) of MnFe2O4 NMs
Fig. S2. Fe and Mn content (a, b), single particle content (c) in tomato leaves, and the
number of NM particles in leaf sap at 0 h (d), 12 h (e), and 72 h (f). The significant
differences between CK and NMs are marked with “*” (p < 0.05).
Fig. S3. Magnetic attraction of chloroplast mixture (left) and chloroplast-NM mixture
(right).
Fig. S4. Flowering time (a), fruit number (b), sugar content (c), net photosynthetic
rate (d), stomatal conductance (e), sucrose content (f) of tomato upon treatment with
different doses of MnFe2O4 NMs. Bars with different letters are significantly
different, and values are mean ± SD (standard deviation). The significant differences
between CK and NMs are marked with “*” (p < 0.05).
Fig. S5. The relative expression of Ferredoxin, PsaA, and PsbA (a), and SPS in
tomato leaves after spraying MnFe2O4 NMs. The significant differences between CK
and NMs are marked with “*” (p < 0.05).
Fig. S6. Phenotype of tomato fruits (a), fruit number (b), fruit fresh weight (c). Bars
with different letters are significantly different, and values are mean ± SD (standard
deviation).
Fig. S7. The companion field experiment to verify the greenhouse effect.
Fig. S8. Partial least squares-discriminate analysis (PLS-DA) score plots of metabolic
profiles of tomato fruits between control and MnFe2O4 NMs.
Fig. S9. Volcano plot of metabolites in control and tomato fruits
upon MnFe2O4 NMs (CK/NMs).
Fig. S10. Sugar-acid ratio (a), and ascorbic acid content (b) in fruit upon MnFe2O4
NMs. The significant differences between CK and NMs are marked with “*” (p <
0.05).
Fig. S11. Relative expression of JA synthesis genes (a), SA synthesis genes (b), and
ethylene synthesis genes (c) in tomato fruits after MnFe2O4 NM application. The
significant differences between CK and NMs are marked with “*” (p < 0.05).
Fig. S12. The auto fluorescence of chlorophyll observed by fluorescence microscopy
in tomato fruit upon control (a) and MnFe2O4 NMs (b), fluorescence intensity of
control and NMs (c).
Fig. S13. Mass of Fe and Mn (a), and P content (b) in tomato fruits upon MnFe2O4
NMs. The significant differences between CK and NMs are marked with “*” (p <
0.05).
Fig. S14 Raw data of the NM determination in tomato fruits by SP-ICP-MS
Table. S1. Fresh/dry weight and root parameters of tomato plants after MnFe2O4 NM
application. The significant differences between CK and NMs are marked with “*” (p
< 0.05).
Table. S2. Differentially expressed metabolites of tomato fruits in CK and upon
MnFe2O4 NMs
Table. S3. Fe and Mn content (mg/g) in fruit in CK and upon MnFe2O4 NMs.
Table S4. Measures of sensitivity, detection limits, and R2 for element detection by
ICP-MS.
Table. S5. Instrumental parameters for SP-ICP-MS data acquisition.
Table. S6. Primer sets list for this study
Gene Primer Sequence (5' to 3')
Actin Forward ATGATAACTCGACGGATCGC
Reverse CTTGGATGTGGTAGCCGT
SPS Forward CGGTGGATGGCAAAACG
Reverse GGCAATCGGCCTCTGGT
Ferredoxin Forward TTCCTCACTCACAATGGCAAC
Reverse CCAGCTCTGTAGTTTTACCTT
PsaA Forward GATTTCTCATAGTTGGTGCTGCTG
Reverse TACAAACCAAAACTGTGAAAGCCT
PsbA Forward CCAAGGTTAGCACGGTTGAT
Reverse CGTAGCCGCTCATGGTTATT
SFT Forward CACCGATATTCCAGCTACCA
Reverse TGTTTGCCGACCTAATTGTC
LeSUT1 Forward TTCCATAGCTGCTGGTGTTC
Reverse TACCAGAAATGGGTCCACAA
LeSUT2 Forward CCTACAGCGTCCCTTTCTCT
Reverse GGATACAACCATCTGAGGTACAA
LIN5 Forward TGAGACTCTGAATGCTTGGAG
Reverse CCATACACACACTTTGCCTTC
LIN7 Forward TGGCAATTAACGACGAGGCACA
Reverse CCCCTTTTACCATAGTTCCTTTCTCCT
GA20ox2 Forward GTGATCCGATTGCAGCTAACGG
Reverse ACGGATGTGCAAGTGAGATAAG
GA20ox3 Forward CTAGTGTTACTAGAGAACTACA
Reverse TGTCAACCCCATGGTTAACCAC
SIGAST1 Forward GCACGTACCGGTGTTCAAAGAC
Reverse CAGTTATTGTAGCAAGGGCAAC
SHT1 Forward AACATCCCTGGAGGAACTTG
Reverse GCATTTGAATTCCACCTTCA
SHT2 Forward TCAACTACGGAACAGCCAAG
Reverse TCAGGTTCAATGTTGTCGGT
SHT3 Forward GTGCTATGAGGTTCGGGATT
Reverse TGGTTGAGTCCATTGGTGTT
LOX13 Forward GGACCACAGATGAAGAACCATTAC
Reverse CTCTGTTCTTCAAGTTCGGATCAT
AOS Forward CGAAACTCCCAGCCCAAAAAG
Reverse ACCTGGTGGCATGTTCGTTC
AOC Forward TTCTACTTCGGCGATTACGGTC
Reverse GGTTAAGTACGCTCCCTGAACG
OPR3 Forward CTTTGAGGAACGCGTATCAGG
Reverse TGACACGAGATCAGCATCACC
PAL5 Forward AACAGCAACATTACCCCGTGTT
Reverse GCAATGTATGACAACGGGACAA
ICS Forward TCATTAGACGATTGGCGTGCTA
Reverse GCTGTTGCATCAAATCGGATT
ACS2 Forward TATGGAGAGTTATTATAAACGATGTTA
Reverse CTAAGTACATAGACCAGTTGTCAATAC
ACS4 Forward ATTCACTAGAGGACTTGAAGAAATAG
Reverse CAAGCTTTATAACTTTATTTGATTGTA
ACO2 Forward AAATTTTGGGACTAAAGTAAGTAACTA
Reverse TAAATTTAGGGTAAACTTGTTTGTTAT
ACO4 Forward GTTACTTGACTTACTCTGTGAAAATCT
Reverse ATATAACTTCATGTAATCATCAAACAC
You might also like
- Gas Chromatography and Mass Spectrometry: A Practical GuideFrom EverandGas Chromatography and Mass Spectrometry: A Practical GuideRating: 5 out of 5 stars5/5 (3)
- Supplemental Information: Keerthi T. Chathoth, J. David Barrass, Shaun Webb, and Jean D. BeggsDocument11 pagesSupplemental Information: Keerthi T. Chathoth, J. David Barrass, Shaun Webb, and Jean D. BeggsJonhny PhamNo ratings yet
- Application of IC-MS and IC-ICP-MS in Environmental ResearchFrom EverandApplication of IC-MS and IC-ICP-MS in Environmental ResearchRajmund MichalskiNo ratings yet
- 5D Structural Characterization of Synthetic PeptidesDocument1 page5D Structural Characterization of Synthetic PeptidesVenkata Suryanarayana GorleNo ratings yet
- Celiker et al 2023_Suppl_Light-responsive microRNA molecules in human retinal organoids are differentially regulated by distinct wavelengths of lightDocument9 pagesCeliker et al 2023_Suppl_Light-responsive microRNA molecules in human retinal organoids are differentially regulated by distinct wavelengths of lightCeren CelayirNo ratings yet
- PaperTrigoHB4Gonzalez Et Al 2019 Fig Compl PDFDocument6 pagesPaperTrigoHB4Gonzalez Et Al 2019 Fig Compl PDFFrancisco AyalaNo ratings yet
- Anti-Inflammatory, Anti-Tumor-Promoting, and Cytotoxic Activities of Constituents of Marigold (Calendula Officinalis) FlowersDocument5 pagesAnti-Inflammatory, Anti-Tumor-Promoting, and Cytotoxic Activities of Constituents of Marigold (Calendula Officinalis) FlowersJoseth Carolina SantanaNo ratings yet
- pmTFP1 FibrillarinDocument4 pagespmTFP1 FibrillarinAlleleBiotechNo ratings yet
- pmTFP1 ZyxinDocument5 pagespmTFP1 ZyxinAlleleBiotechNo ratings yet
- pmTFP1 ActinDocument4 pagespmTFP1 ActinAlleleBiotechNo ratings yet
- Pmwasabi FibrillarinDocument5 pagesPmwasabi FibrillarinAlleleBiotechNo ratings yet
- Part 6 Mutation Winter 2023Document2 pagesPart 6 Mutation Winter 2023tanusehdev17No ratings yet
- ExercisesDocument6 pagesExercisesDiego ForeroNo ratings yet
- Assembly of A Biocompatible Triazole-Linked Gene by One-Pot click-DNA Ligation. Suplementary InfomrationDocument15 pagesAssembly of A Biocompatible Triazole-Linked Gene by One-Pot click-DNA Ligation. Suplementary Infomrationdobrovolskis.bioNo ratings yet
- Supplemental Data Induction of Pluripotent Stem Cells From Mouse Embryonic and Adult Fibroblast Cultures by Defined FactorsDocument41 pagesSupplemental Data Induction of Pluripotent Stem Cells From Mouse Embryonic and Adult Fibroblast Cultures by Defined FactorsewrjdNo ratings yet
- 8GOMDocument6 pages8GOMaakif inayatNo ratings yet
- Studies On Manganese (Ii) Catalyzed Oxidation of N-Methylaniline by Periodate IonDocument10 pagesStudies On Manganese (Ii) Catalyzed Oxidation of N-Methylaniline by Periodate IonYuda AryokoNo ratings yet
- Trower, 1996. in Vitro Mutagenesis Protocols Volume 57Document9 pagesTrower, 1996. in Vitro Mutagenesis Protocols Volume 57afinaNo ratings yet
- pmTFP1 MitochondriaDocument4 pagespmTFP1 MitochondriaAlleleBiotechNo ratings yet
- 1995 - NAR - Solid-Phase Revesible Immobilization For The Isolation of PCR ProductsDocument2 pages1995 - NAR - Solid-Phase Revesible Immobilization For The Isolation of PCR ProductsKhoa NguyendangNo ratings yet
- Table2 PDFDocument1 pageTable2 PDFvaleriaovandoNo ratings yet
- pmTFP1 PeroxisomesDocument4 pagespmTFP1 PeroxisomesAlleleBiotechNo ratings yet
- All Ustrious Pmwasabi AnnexinDocument4 pagesAll Ustrious Pmwasabi AnnexinAlleleBiotechNo ratings yet
- pmTFP1 ClathrinDocument4 pagespmTFP1 ClathrinAlleleBiotechNo ratings yet
- Detection of Sulfur Compounds in Natural GasDocument4 pagesDetection of Sulfur Compounds in Natural GasfarshidianNo ratings yet
- Practice Final Exam - Answer KeyDocument9 pagesPractice Final Exam - Answer KeyGloria LamNo ratings yet
- Bacterias Del DesiertoDocument73 pagesBacterias Del DesiertoBrenda ValoNo ratings yet
- AMPK and TOR (2020)Document21 pagesAMPK and TOR (2020)BastianRiverosVasquezNo ratings yet
- Synthesis of Antitumor 6-Alkylidenepenicillanate Sulfones and Related 3-Alkylidene-2-AzetidinonesDocument4 pagesSynthesis of Antitumor 6-Alkylidenepenicillanate Sulfones and Related 3-Alkylidene-2-AzetidinonesBedanta BorahNo ratings yet
- JCM 32 4 1095-1098 1994Document4 pagesJCM 32 4 1095-1098 1994mariotecNo ratings yet
- 619e4d83b653b Biology Genetics Laboratory Exercise The Central Dogma of GeneticsDocument10 pages619e4d83b653b Biology Genetics Laboratory Exercise The Central Dogma of GeneticsKimNo ratings yet
- Subunidad SigmaDocument5 pagesSubunidad SigmaGeorgina HernandezNo ratings yet
- Pnas 1621154114 SappDocument16 pagesPnas 1621154114 SappAlison Raquel Hernández AvelarNo ratings yet
- pmTFP1 Cx26Document4 pagespmTFP1 Cx26AlleleBiotechNo ratings yet
- Bioinformatics LAb ReportDocument7 pagesBioinformatics LAb ReportBriana Halbert100% (3)
- RegRNA - A Regulatory RNA Motifs and Elements FinderDocument2 pagesRegRNA - A Regulatory RNA Motifs and Elements FinderBiotechGenieNo ratings yet
- Y Chromosomal Haplogroup Genotyping Using SNP AnalysisDocument3 pagesY Chromosomal Haplogroup Genotyping Using SNP AnalysisIfetayo Osifeso-FakoredeNo ratings yet
- De. Kaleem AhmadDocument5 pagesDe. Kaleem AhmadTucker LambertNo ratings yet
- 1990 - White TJ Et Al., AMPLIFICATION AND DIRECT SEQUENCING OF GUNGAL RIBOSOMAL RNA GENES FOR PHYLOGENETICSDocument8 pages1990 - White TJ Et Al., AMPLIFICATION AND DIRECT SEQUENCING OF GUNGAL RIBOSOMAL RNA GENES FOR PHYLOGENETICSsararmentaNo ratings yet
- SMB 1Document7 pagesSMB 1Subhecchha BaidyaNo ratings yet
- Postharvest Biotechnology Prospects at King Mongkut's University of Technology ThonburiDocument64 pagesPostharvest Biotechnology Prospects at King Mongkut's University of Technology ThonburiintanrosalinaNo ratings yet
- Primers For TKO Library NGSDocument1 pagePrimers For TKO Library NGSfmachour9316No ratings yet
- Nar 00609 H 2013 File006Document9 pagesNar 00609 H 2013 File006Ronilo Jose Danila FloresNo ratings yet
- Nqx013 SuppDocument4 pagesNqx013 SuppCamilofonoNo ratings yet
- pmTFP1 Cx32Document4 pagespmTFP1 Cx32AlleleBiotechNo ratings yet
- Molecular Biology-Transcription and Translation: Recitation Section 8Document8 pagesMolecular Biology-Transcription and Translation: Recitation Section 8Constantine David AkritidesNo ratings yet
- pmTFP1 VSV GDocument4 pagespmTFP1 VSV GAlleleBiotechNo ratings yet
- Id Converters TestDocument8 pagesId Converters Testsilico-hmgNo ratings yet
- Analysis of The 3-Glycidoxypropyltrimethoxysilane (GPTMS) Hydrolysis by Infrared SpectrosDocument11 pagesAnalysis of The 3-Glycidoxypropyltrimethoxysilane (GPTMS) Hydrolysis by Infrared SpectrosMosNo ratings yet
- PCA For Removal of Noise PCA For Removal of Noise: GC/MS ExampleDocument9 pagesPCA For Removal of Noise PCA For Removal of Noise: GC/MS Exampledéborah_rosalesNo ratings yet
- pmTFP1 VimentinDocument4 pagespmTFP1 VimentinAlleleBiotechNo ratings yet
- Bio IC Lesson 7 - MutationDocument13 pagesBio IC Lesson 7 - MutationKurt KleinNo ratings yet
- Supplementary Materials: Chun Kit Kwok, Yiliang Ding, Madeline E. Sherlock, Sarah M. Assmann, Philip C. BevilacquaDocument9 pagesSupplementary Materials: Chun Kit Kwok, Yiliang Ding, Madeline E. Sherlock, Sarah M. Assmann, Philip C. BevilacquasuryasanNo ratings yet
- Ammonium Sulphate Functions in PCRDocument12 pagesAmmonium Sulphate Functions in PCRĐặng Gia HoàngNo ratings yet
- Nature02358 s1Document11 pagesNature02358 s1Nimas GhasaniNo ratings yet
- Exercises Genetics USTH2022Document15 pagesExercises Genetics USTH2022yungiang157No ratings yet
- Molecular Cloning and Homology Modeling of Novel Tyro - 2016 - Achievements in TDocument7 pagesMolecular Cloning and Homology Modeling of Novel Tyro - 2016 - Achievements in Tsameer sahaanNo ratings yet
- Supplementary Materials ForDocument21 pagesSupplementary Materials ForNguyen Linh NhamNo ratings yet
- Assignment 2Document2 pagesAssignment 2Sarah MocksNo ratings yet
- Sannaangotzi 2017Document12 pagesSannaangotzi 2017Yassine MOUHIBNo ratings yet
- Ma 2021Document10 pagesMa 2021Yassine MOUHIBNo ratings yet
- Fu 2021Document17 pagesFu 2021Yassine MOUHIBNo ratings yet
- Jiang 2016Document27 pagesJiang 2016Yassine MOUHIBNo ratings yet
- The Role of Strategies in SuccessDocument2 pagesThe Role of Strategies in SuccessGeorge DelacruzNo ratings yet
- Insight IAS Prelims 2020 Test 13 S PDFDocument66 pagesInsight IAS Prelims 2020 Test 13 S PDFApooNo ratings yet
- English written preparation for Dhaka University admission test (DUDocument7 pagesEnglish written preparation for Dhaka University admission test (DUShanian Ahmed100% (3)
- Talking About ProfessionsDocument17 pagesTalking About ProfessionsenglishcommunityworkNo ratings yet
- Aerated Grit Chamber Design ParametersDocument26 pagesAerated Grit Chamber Design ParametersMarc NguyenNo ratings yet
- Unit 1 Cultural Issues and ValuesDocument3 pagesUnit 1 Cultural Issues and ValuesACHRAF DOUKARNENo ratings yet
- 3 - Green Synthesis of A Fluorescent Natural Product PDFDocument3 pages3 - Green Synthesis of A Fluorescent Natural Product PDFjavier roo ror100% (1)
- Chemistry April 14 Reading Essentials - Properties of MatterDocument6 pagesChemistry April 14 Reading Essentials - Properties of MatterAljhon A. MarajuniNo ratings yet
- Sources List for Canadian Atlas DataDocument12 pagesSources List for Canadian Atlas DataMohamed JalalNo ratings yet
- Probability and Statistics ExamDocument4 pagesProbability and Statistics ExamLeul SolomonNo ratings yet
- RAN - Installation MoP-v01 - Safaricom Ethiopia - V1.1 - 08122021Document32 pagesRAN - Installation MoP-v01 - Safaricom Ethiopia - V1.1 - 08122021Tsehay MisrakNo ratings yet
- Rising Strong: How The Ability To Reset Transforms The Way We Live, Love, Parent, and Lead - Brené BrownDocument5 pagesRising Strong: How The Ability To Reset Transforms The Way We Live, Love, Parent, and Lead - Brené Browndarupasi40% (5)
- International Application InstructionsDocument4 pagesInternational Application InstructionsTahiNo ratings yet
- Magnetic Particle Test Record Truck Crane Articulating Boom (Gb-La03)Document2 pagesMagnetic Particle Test Record Truck Crane Articulating Boom (Gb-La03)Hario PramuditoNo ratings yet
- Activities guide and evaluation rubric - Unit 3 - Task 4 - Oral Production - Voices in Motion (1)Document7 pagesActivities guide and evaluation rubric - Unit 3 - Task 4 - Oral Production - Voices in Motion (1)Darleison VergelNo ratings yet
- Measuring Solar Radiation the Right WayDocument14 pagesMeasuring Solar Radiation the Right WayObada Ar-ruzziNo ratings yet
- Kelas Xii - May I Help You - Chapter 1Document2 pagesKelas Xii - May I Help You - Chapter 1Syifa Fauziah50% (2)
- Coriolis Mass Flow MeterDocument2 pagesCoriolis Mass Flow MeterSreejesh SundaresanNo ratings yet
- Local Media3854443116550985649Document17 pagesLocal Media3854443116550985649Mediado Karen TomenioNo ratings yet
- Formation en Coaching Integral These de BAKALADocument6 pagesFormation en Coaching Integral These de BAKALAHamza ZERBONo ratings yet
- SurveyingDocument14 pagesSurveyingkNo ratings yet
- UntitledDocument19 pagesUntitledSmiles PrintingNo ratings yet
- Valvoline Lithium Ep2 GreaseDocument1 pageValvoline Lithium Ep2 GreaseDicky PratamaNo ratings yet
- Square PharmaceuticalsDocument2 pagesSquare PharmaceuticalsAl NomanNo ratings yet
- Cambridge Primary Reading Student's Book 6Document13 pagesCambridge Primary Reading Student's Book 6Mari Gold0% (1)
- Guide For The Use of Educational Resources - Software Smith V4.0Document7 pagesGuide For The Use of Educational Resources - Software Smith V4.0Sebastián Barona VélezNo ratings yet
- Gap Year Thesis StatementDocument4 pagesGap Year Thesis Statementbrookelordmanchester100% (1)
- Dian Medisa, Hady Anshory, Putri Litapriani, Rezky Fajriyati MDocument9 pagesDian Medisa, Hady Anshory, Putri Litapriani, Rezky Fajriyati MNada LathifahNo ratings yet
- Course-Outline-For-Stud2020 (1) - English 9 THIRD QUARTERDocument3 pagesCourse-Outline-For-Stud2020 (1) - English 9 THIRD QUARTERTyrone Dave BalitaNo ratings yet