Professional Documents
Culture Documents
Reagents 1
Reagents 1
Uploaded by
Ananya Sharma0 ratings0% found this document useful (0 votes)
2 views2 pagesThe document describes various reagents and their functions in organic chemistry reactions. Sodium or potassium hydroxide is used to hydrolyze haloalkanes into alcohols. Silver nitrate in ammonia tests for the presence of halides in compounds by forming precipitates. Zinc in acetic acid performs the Clemmensen reduction of ketones to alkanes. Hydrogen in the presence of a catalyst reduces haloalkanes to alkanes.
Original Description:
Original Title
REAGENTS 1
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document describes various reagents and their functions in organic chemistry reactions. Sodium or potassium hydroxide is used to hydrolyze haloalkanes into alcohols. Silver nitrate in ammonia tests for the presence of halides in compounds by forming precipitates. Zinc in acetic acid performs the Clemmensen reduction of ketones to alkanes. Hydrogen in the presence of a catalyst reduces haloalkanes to alkanes.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
2 views2 pagesReagents 1
Reagents 1
Uploaded by
Ananya SharmaThe document describes various reagents and their functions in organic chemistry reactions. Sodium or potassium hydroxide is used to hydrolyze haloalkanes into alcohols. Silver nitrate in ammonia tests for the presence of halides in compounds by forming precipitates. Zinc in acetic acid performs the Clemmensen reduction of ketones to alkanes. Hydrogen in the presence of a catalyst reduces haloalkanes to alkanes.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
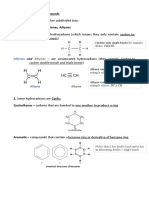
REAGENTS AND THEIR FUNCTIONS
CHAPTER 1 - ALKYL AND ARYL HALIDES
1. **Sodium or Potassium Hydroxide (NaOH/KOH):**
- **Function:** Used in the hydrolysis of haloalkanes to form alcohols.
- **Example:** CH3CH2Cl + NaOH → CH3CH2OH + NaCl
2. **Silver Nitrate (AgNO3) in Ammonia (NH3):**
- **Function:** Used to test the presence of halides (Cl, Br, I) in organic compounds.
- **Example:** C6H5Cl + AgNO3/NH3 → C6H5AgCl (white precipitate)
3. **Zinc (Zn) in Acetic Acid (CH3COOH):**
- **Function:** Used in the Clemmensen reduction to convert ketones to alkanes.
- **Example:** CH3COCH3 + Zn/CH3COOH → CH3CH3
4. **Hydrogen (H2) in the Presence of a Catalyst (e.g., Pd, Pt, Ni):**
- **Function:** Used for the reduction of haloalkanes to form alkanes.
- **Example:** CH3CH2Cl + H2/Pd → CH3CH3
5. **Alcoholic KOH (Potassium Hydroxide):**
- **Function:** Used in the Williamson synthesis for the preparation of ethers.
- **Example:** C2H5OH + KOH → C2H5O^-K+ + H2O
6. **NaI in Acetone:**
- **Function:** Used in the Finkelstein reaction to convert haloalkanes to alkyl iodides.
- **Example:** CH3CH2Br + NaI/Acetone → CH3CH2I + NaBr
7. **Aluminum Chloride (AlCl3):**
- **Function:** Used as a Lewis acid catalyst in Friedel-Crafts alkylation and acylation reactions of benzene.
- **Example:** C6H6 + CH3Cl/AlCl3 → C6H5CH3 (Toluene)
8. **Sodium Amide (NaNH2):**
- **Function:** Used in the Hofmann bromamide degradation reaction to convert primary amides to primary
amines.
- **Example:** CH3CONH2 + Br2/NaNH2 → CH3NH2 + NaBr + Na2CO3
9. **Conc. H2SO4 (Sulfuric Acid):**
- **Function:** Used in the dehydration of alcohols to form alkenes.
- **Example:** CH3CH2OH + H2SO4 → CH2=CH2 + H2O
10. **Sodium in Liquid Ammonia (Birch Reduction):**
- **Function:** Used for the reduction of unsaturated compounds, like benzene rings.
- **Example:** C6H6 + 2Na/NH3 → C6H12 (Cyclohexane) + NaNH2
11. **Concentrated Hydrochloric Acid (HCl):**
- **Function:** Used in the hydrolysis of haloalkanes to form alkyl halides.
- **Example:** CH3CH2Cl + HCl → CH3CH2Cl + H2O
12. **Conc. Sulfuric Acid (H2SO4):**
- **Function:** Used for the dehydration of alcohols to form alkenes.
- **Example:** CH3CH2CH2OH + H2SO4 → CH3CH=CH2 + H2O
13. **Sodium Metal (Na) or Grignard Reagent (RMgX):**
- **Function:** Used to prepare alkanes, alkenes, or alkynes from haloalkanes.
- **Example:** CH3Cl + 2Na → CH4 + 2NaCl
14. **Sodium Bicarbonate (NaHCO3):**
- **Function:** Used to neutralize acids and remove acidic impurities.
- **Example:** CH3COOH + NaHCO3 → CH3COONa + H2O + CO2
15. **Phosphorus Pentachloride (PCl5) and Phosphorus Trichloride (PCl3):**
- **Function:** Used for the conversion of alcohols to alkyl halides.
- **Example:** CH3CH2OH + PCl5 → CH3CH2Cl + HCl + POCl3
16. **Fuming Nitric Acid (HNO3):**
- **Function:** Used in the nitration of aromatic compounds (e.g., benzene).
- **Example:** C6H6 + HNO3 → C6H5NO2 + H2O
17. **Hydrobromic Acid (HBr) with Peroxide (H2O2):**
- **Function:** Used in the radical addition of HBr to alkenes to form alkyl bromides. The peroxide (H2O2) is
often used as a radical initiator.
- **Example:** CH2=CH2 + HBr/H2O2 → CH3CH2Br
18. **Bromine (Br2) in CCl4:**
- **Function:** Used in the addition reaction to test the unsaturation of organic compounds.
- **Example:** C2H4 + Br2/CCl4 → C2H4Br2
You might also like
- Sulfuric Acid Manufacture: Analysis, Control and OptimizationFrom EverandSulfuric Acid Manufacture: Analysis, Control and OptimizationRating: 3.5 out of 5 stars3.5/5 (3)
- Experiment 7 - HydrocarbonsDocument7 pagesExperiment 7 - HydrocarbonsChristine DomingoNo ratings yet
- Organic II Reactions (Complete) BETADocument21 pagesOrganic II Reactions (Complete) BETATheoNo ratings yet
- Ib Chemistry SL Chapter 10 PDF FreeDocument21 pagesIb Chemistry SL Chapter 10 PDF FreeJustin MatthewNo ratings yet
- Orgo Reaction SheetDocument9 pagesOrgo Reaction SheetKyle Broflovski100% (1)
- Nitric Acid Production PDFDocument87 pagesNitric Acid Production PDFmohamedNo ratings yet
- UTAR Chem Lab 1 Short Report Exp7Document4 pagesUTAR Chem Lab 1 Short Report Exp7Izykiel EdwardNo ratings yet
- Chemistry HSC FormulasDocument6 pagesChemistry HSC Formulashpgc101100% (1)
- Xii Chem Prep. Paper 2023Document3 pagesXii Chem Prep. Paper 2023HiraNo ratings yet
- Xii-Chem-Chptr-4-P-Block ElementsDocument69 pagesXii-Chem-Chptr-4-P-Block ElementsTanveer AhmedNo ratings yet
- Organic II Reactions BETADocument8 pagesOrganic II Reactions BETARicky Fontaine100% (9)
- Carboxylic Acids and DerivativesDocument5 pagesCarboxylic Acids and DerivativesahumanbeinginearthNo ratings yet
- Aluminum Oxide From Bayer Process, Metallurgical GradeDocument28 pagesAluminum Oxide From Bayer Process, Metallurgical GradeSeyit AvcuNo ratings yet
- Organic Chemistry I: The Unofficial Reaction SheetDocument11 pagesOrganic Chemistry I: The Unofficial Reaction SheetKarl WilsonNo ratings yet
- R - IGCSE Resources - Chemistry Revision Guide by MohamedDocument13 pagesR - IGCSE Resources - Chemistry Revision Guide by MohamedShubam PandeyNo ratings yet
- Module 5 - XII NEET - ChemistryDocument128 pagesModule 5 - XII NEET - ChemistryGhanshyam MatlaneNo ratings yet
- Naming ReactionDocument8 pagesNaming Reactionsharayu2406No ratings yet
- Manufactures in Industry and Chemical For ConsumerDocument22 pagesManufactures in Industry and Chemical For ConsumerMuhammad DanishNo ratings yet
- Amines Bounceback One ShotDocument100 pagesAmines Bounceback One ShotShivadeep Vishwakarma0% (1)
- c06s02 PDFDocument6 pagesc06s02 PDFDewiRSNo ratings yet
- Chapter 3 ConceptsDocument4 pagesChapter 3 ConceptsEmiliaFigueroaAizpurúaNo ratings yet
- 122 CH 9 AldehydesandketonesDocument50 pages122 CH 9 AldehydesandketonesChemistNo ratings yet
- All Chemical ReactionsDocument29 pagesAll Chemical ReactionsManeet SinghNo ratings yet
- Lampiran A Sudah FinalDocument20 pagesLampiran A Sudah FinalBayu Handika PrasetyoNo ratings yet
- Plant Design For The Production of Sodium CarbonateDocument29 pagesPlant Design For The Production of Sodium CarbonateMulugeta Getenet83% (6)
- 17 Organic Chemistry Alkyl HalidesDocument15 pages17 Organic Chemistry Alkyl HalidesAtul VermaNo ratings yet
- Chemistry Project: Name: Azad Abdullahi Class: Ss3S Teacher: Mr. AdekunleDocument14 pagesChemistry Project: Name: Azad Abdullahi Class: Ss3S Teacher: Mr. Adekunleapi-383198550% (2)
- Chemistry-Folio Form 4Document45 pagesChemistry-Folio Form 4Ahmad Izzat Mohd HanafiNo ratings yet
- Exp 1Document9 pagesExp 1Lolo OmarNo ratings yet
- Chemical Process Industries: Industrial GasesDocument5 pagesChemical Process Industries: Industrial GasesjantskieNo ratings yet
- Kimia Chapter 9Document35 pagesKimia Chapter 9Mohammad AmirNo ratings yet
- Wiki Hidrolisis Dan Kco3Document3 pagesWiki Hidrolisis Dan Kco3Gift GiftNo ratings yet
- ChemistryDocument11 pagesChemistryEttaNo ratings yet
- Table 1: Elements Symbols Atomic MassDocument7 pagesTable 1: Elements Symbols Atomic MassMadhavNo ratings yet
- Historical ProfileDocument20 pagesHistorical Profilechandan.cj99No ratings yet
- CHEMISTRY-XII 2022 June 3rd PrelimDocument2 pagesCHEMISTRY-XII 2022 June 3rd PrelimSauban AhmedNo ratings yet
- Gases Serve As Anesthetics Liquid Alkanes Light Liquids Harmful To Lungs Heavy Liquids Mineral Oil Petroleum JellyDocument23 pagesGases Serve As Anesthetics Liquid Alkanes Light Liquids Harmful To Lungs Heavy Liquids Mineral Oil Petroleum JellyFilipe Gama FreireNo ratings yet
- All Chemical Reactions 2023Document29 pagesAll Chemical Reactions 2023Aryan MishraNo ratings yet
- AK - Carbonyl CompoundDocument8 pagesAK - Carbonyl Compoundmgupta13marNo ratings yet
- Aliphatic XIIDocument45 pagesAliphatic XIISUYOG K.C.No ratings yet
- Capitulo, Korben Philson A. February 3, 2017 CE-3A Environmental Engineering Assignment No. 4Document2 pagesCapitulo, Korben Philson A. February 3, 2017 CE-3A Environmental Engineering Assignment No. 4Korbs CapituloNo ratings yet
- General Organic ChemistryDocument10 pagesGeneral Organic ChemistryRiddhi Chatterjee100% (2)
- 45 Hydrocarbons AlkanesDocument7 pages45 Hydrocarbons Alkanessujalgupta0123456789No ratings yet
- 1.1 ObjectiveDocument5 pages1.1 Objectivemuhammad hafizuddinNo ratings yet
- Extraction of AluminiumDocument3 pagesExtraction of AluminiumuniquestarNo ratings yet
- Extraction of Aluminium From BauxiteDocument7 pagesExtraction of Aluminium From BauxiteMAKNNIE KPOPNo ratings yet
- Chapter-Wise Important Chemical Reactions For Class 10Document9 pagesChapter-Wise Important Chemical Reactions For Class 10Manish SainNo ratings yet
- Uses of Sulphuric AcidDocument14 pagesUses of Sulphuric AcidFaizul FaiiziNo ratings yet
- Text 2didididDocument3 pagesText 2didididhussainshakeel.mzNo ratings yet
- Annex 3.2 Industrial Processes Sector-Ammonia Production-Kellog Process Detailed Description PDFDocument5 pagesAnnex 3.2 Industrial Processes Sector-Ammonia Production-Kellog Process Detailed Description PDFErol DAĞNo ratings yet
- Organic Mind MapDocument37 pagesOrganic Mind Mapkamalia8980% (5)
- Hydroxy CompoundsDocument7 pagesHydroxy CompoundsahumanbeinginearthNo ratings yet
- Organic ReactionsDocument4 pagesOrganic ReactionsRobbing_HoodNo ratings yet
- Inorganic Chemistry Report PLCDocument10 pagesInorganic Chemistry Report PLCPablo LópezNo ratings yet
- Acid BaseDocument18 pagesAcid BasechaitanyaNo ratings yet
- Acfrogcxqpd3dqml GQHMZQ B0c089di81vpcrvgphrwcu4gh Bzezvshldjt4clxztdrke4cieuxds1wlvk6scla 0byn2rmeu4btdaq8ckybm0chweegnztu7u2olcnli2lia5txmf386nyikuDocument6 pagesAcfrogcxqpd3dqml GQHMZQ B0c089di81vpcrvgphrwcu4gh Bzezvshldjt4clxztdrke4cieuxds1wlvk6scla 0byn2rmeu4btdaq8ckybm0chweegnztu7u2olcnli2lia5txmf386nyikuAchal ParekhNo ratings yet
- CAIE Chemistry A-Level: 14: HydrocarbonsDocument9 pagesCAIE Chemistry A-Level: 14: HydrocarbonsahumanbeinginearthNo ratings yet
- Silicon in Organic Synthesis: Butterworths Monographs in Chemistry and Chemical EngineeringFrom EverandSilicon in Organic Synthesis: Butterworths Monographs in Chemistry and Chemical EngineeringNo ratings yet
- Alkane FunctionalizationFrom EverandAlkane FunctionalizationArmando J. L. PombeiroNo ratings yet
- Bpo C Chapter 10Document55 pagesBpo C Chapter 10DewiSugiartiNo ratings yet
- Alkanes and CycloalkanesDocument63 pagesAlkanes and Cycloalkanesayunna ayunniNo ratings yet
- Year 10 Chemistry Revision Schedule BookletDocument8 pagesYear 10 Chemistry Revision Schedule BookletDermot ChuckNo ratings yet
- Class XII - PCM - Syllabus 2024-25Document2 pagesClass XII - PCM - Syllabus 2024-25Shubham KumarNo ratings yet
- Cahn Ingold PrelogDocument13 pagesCahn Ingold PrelogVivien TeyNo ratings yet
- Download pdf General Organic Biological Chemistry Third Edition Janice G Smith ebook full chapterDocument53 pagesDownload pdf General Organic Biological Chemistry Third Edition Janice G Smith ebook full chapterlouise.sanchez601100% (1)
- Magnesium in Methanol (MG - MeOH) in Organic Syntheses (Current Organic Chemistry, 2004, 8, 13, 1263-1287) PDFDocument26 pagesMagnesium in Methanol (MG - MeOH) in Organic Syntheses (Current Organic Chemistry, 2004, 8, 13, 1263-1287) PDFDmitryNo ratings yet
- Linear Alkylbenzene ProductionDocument20 pagesLinear Alkylbenzene ProductionAhmed AtefNo ratings yet
- Chapter 01 2SPPDocument46 pagesChapter 01 2SPPGheorghe-Lucian IrimiaNo ratings yet
- Preboard Answer Key 1 PDFDocument11 pagesPreboard Answer Key 1 PDFAnonymous 0zrCNQNo ratings yet
- AlkaneDocument6 pagesAlkaneJue MayaNo ratings yet
- Aldehydes and KetonesDocument26 pagesAldehydes and KetonesLuckyMirandaNo ratings yet
- 08 Chapter 12Document26 pages08 Chapter 12M Zia DogarNo ratings yet
- Families of Organic CompoundsDocument8 pagesFamilies of Organic CompoundsJessa Mae LangcuyanNo ratings yet
- Modeling of Adiabatic Movingbed Reactor For Dehydrogenation of Isobutane To IsobuteneDocument7 pagesModeling of Adiabatic Movingbed Reactor For Dehydrogenation of Isobutane To IsobuteneForcus onNo ratings yet
- Chapter 9 Lecture PDFDocument128 pagesChapter 9 Lecture PDFjoseph changNo ratings yet
- Carbonyl Chemistry Tutorial #8 2018-2019 AnswersDocument6 pagesCarbonyl Chemistry Tutorial #8 2018-2019 AnswersZoe NorvilleNo ratings yet
- Alkenes and Alkynes: Lesson 3Document21 pagesAlkenes and Alkynes: Lesson 3AlbaraaAliNo ratings yet
- Mu Et Al 2024 Shape Selectivity of Ael Channels For Anomalously Facilitating Biojet Fuel Production From Long Chain NDocument11 pagesMu Et Al 2024 Shape Selectivity of Ael Channels For Anomalously Facilitating Biojet Fuel Production From Long Chain Nrozsor2100% (1)
- Chemical ManufactureDocument18 pagesChemical ManufactureChellam Siva Chellam Siva100% (1)
- Chemistry Pase-II MDCAT Paper 2017Document25 pagesChemistry Pase-II MDCAT Paper 2017Aarish KhanNo ratings yet
- Chemistry Higher 2Document16 pagesChemistry Higher 2Zach EganNo ratings yet
- 86 TricksDocument164 pages86 Trickslozerface8863% (8)
- Chemistry Art Integration Project: Nomenclature and Isomerism in Organic CompoundsDocument21 pagesChemistry Art Integration Project: Nomenclature and Isomerism in Organic CompoundsPriyank YadavNo ratings yet
- Patent Application Publication (10) Pub. No.: US 2009/0192234 A1Document26 pagesPatent Application Publication (10) Pub. No.: US 2009/0192234 A1Deva DrillTechNo ratings yet
- Experiment 6 Organic ChemistryDocument11 pagesExperiment 6 Organic Chemistryjun keat tanNo ratings yet
- Chemistry Notes by Vasumitra GajbhiyeDocument41 pagesChemistry Notes by Vasumitra GajbhiyeYash Asudani100% (2)
- Page 1 of 40Document61 pagesPage 1 of 40Garima KapoorNo ratings yet