Professional Documents
Culture Documents
Lipid
Uploaded by
Amjad AlmousawiOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Lipid
Uploaded by
Amjad AlmousawiCopyright:
Available Formats
why formed micelles in the absorption of lipid?
The products of lipid digestion, such as fatty acids, monoglycerides, and glycerol, are not very
soluble. This means they do not dissolve well in water, which is a problem because the
environment in the small intestine is aqueous
To overcome this, the monoglycerides and fatty acids associate with phospholipids and bile salts
to form micelles12. These are very small droplets that aid in the transport of these molecules to
the surface of the epithelial cells12.
Micelles are lipoproteins designed for the transport of lipids3. They facilitate absorption by
microvilli, where the fatty acids and proteins diffuse out to form lipoproteins3.
The micelles break down and add to a pool of fatty acids and monoglycerides that are dissolved
in the small intestine solution surrounding the epithelial cells1. These freely dissolved molecules
enter the epithelial cell by diffusion1.
So, micelles are integral to lipid absorption because they allow the lipids to be transported in an
aqueous environment, and they facilitate the absorption of these lipids into the epithelial cells of
the small intestine
What is the purpose of the lack of the glycerol kinase enzyme in adipose tissues?
Adipose tissue serves as a major energy reservoir in the body. By releasing glycerol and fatty
acids into the bloodstream during times of energy demand, adipose tissue contributes to the
overall energy homeostasis of the organism. The absence of glycerol kinase in adipose tissue
ensures that released glycerol is not reincorporated into triglycerides within the adipocytes,
facilitating its availability for energy production in other tissues.
which is likely the reason that triacylglycerols rather than glycogen were selected in evolution as
the major energy reservoir?
بسبب الطاقة العالية
وبسبب قوة سحب الماء للكلوكوز
Why gastric and lingual lipase are limited?
• No emulsification of fats takes place in stomach
• The enzyme secreted in small quantity
• pH of gastric juice is not conducive which is highly acidic
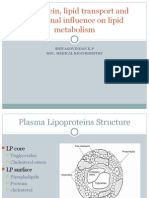
already discussed. Apolipoprotein B (apo B) plays an important role in the transport of
triacylglycerols and cholesterol by forming an amphipathic spherical shell around the
lipids carried in lipoprotein particles . Apo B exists in two forms,
The larger form, synthesized by the liver.
The smaller form, synthesized by the small intestine.
Apo B-48 contains the 2152(240-kDa) N-terminal residues of the 4536-residue(512-kDa) apo B-
100. This truncated molecule can form lipoprotein particles but cannot bind to the low-density-
lipoprotein receptor on cell surfaces.
What is the biosynthetic relation of these two forms of apo B? One possibility a priori is that apo
B-48 is produced by proteolytic cleavage of apo B-100, and another is that the two forms arise
from alternative splicing . The results of experiments show that neither occurs. A totally
unexpected and new mechanism for generating diversity is at work: the changing of the
nucleotide sequence of mRNA after its synthesis . A specific cytidine residue of mRNA is
deaminated to uridine, which changes the codon at residue 2153 from CAA (Gln) to UAA (stop).
The deaminase that catalyzes this reaction is present in the small intestine, but not in the liver,
and is expressed only at certain developmental stages
why Gastric lipase activity requires presence of Ca++?
the activity of many lipases is influenced by Ca++. Calcium ions can stabilize the structure of the enzyme,
enhance its resistance to changes in pH and temperature
Calcium ions can engage in electrostatic interactions with charged amino acid residues on the enzyme
surface
? بعدم وجود االنسولينLPL ففي حاله الصيام كيف يتفعلCII اذا كان االنسولين هو يحفز
Bile acids reversibly form thermodynamically stable aggregates, called micelles
what the effector of lipoprotein lipase activity?
1. Apolipoprotein C-II (apoC-II):
ApoC-II is an activator of lipoprotein lipase. It is a cofactor that associates
with LPL and enhances its catalytic activity. ApoC-II is often found on the
surface of chylomicrons and very-low-density lipoproteins (VLDL) and
stimulates LPL-mediated hydrolysis of their triglycerides.
2. Heparan Sulfate Proteoglycans (HSPG):
LPL is anchored to the endothelial surface by interacting with heparan
sulfate proteoglycans. These proteoglycans serve as cofactors for LPL
activity by presenting lipoprotein substrates to the enzyme. The interaction
with HSPG is important for the efficient hydrolysis of lipoprotein
triglycerides.
3. Insulin:
Insulin is a hormone that enhances the activity of lipoprotein lipase. Insulin
promotes the synthesis and release of lipoprotein lipase from cells,
increasing the enzyme's availability. It also stimulates the uptake of fatty
acids into cells, promoting their storage as triglycerides.
4. Adipose Tissue Hormones:
Hormones released by adipose (fat) tissue, such as adiponectin, can
influence lipoprotein lipase activity. Adiponectin, for example, has been
shown to enhance the expression and activity of LPL.
5. Postprandial State (Fed State):
LPL activity is generally increased in the postprandial (fed) state when
there is an increased need for the hydrolysis of dietary triglycerides. The
presence of chylomicrons and VLDL in the bloodstream during this state
stimulates LPL activity.
6. Fasting State:
Conversely, during fasting, when the body needs to mobilize stored
energy, lipoprotein lipase activity may be reduced. This is part of the
regulatory mechanism to balance energy utilization and storage
You might also like
- Guc 2705 59 28561 2023-05-18T10 45 27Document47 pagesGuc 2705 59 28561 2023-05-18T10 45 27malak00mohamed229No ratings yet
- Digestion and Absorption of LipidsDocument5 pagesDigestion and Absorption of LipidsAngelo Jude Cobacha100% (1)
- BCHEM 254 Metabolism of Nutrients II-Lecture 1 20180121-1Document140 pagesBCHEM 254 Metabolism of Nutrients II-Lecture 1 20180121-1Nicholas BoampongNo ratings yet
- FA MetabolismDocument23 pagesFA MetabolismMaham KhanNo ratings yet
- 1 Lipids Met ИсправDocument54 pages1 Lipids Met ИсправSharikNo ratings yet
- Name: Geonyzl L. Alviola Date: September 09, 2016 Subject: Chemical PhysiologyDocument38 pagesName: Geonyzl L. Alviola Date: September 09, 2016 Subject: Chemical PhysiologyMeeshaNo ratings yet
- Lipids Digestion and AbsorptionDocument28 pagesLipids Digestion and AbsorptionEng Matti Ur RehmanNo ratings yet
- Lipoprotein MetabolismDocument23 pagesLipoprotein MetabolismDarien LiewNo ratings yet
- Ch15 Metabolism of Dietary LipidsDocument37 pagesCh15 Metabolism of Dietary LipidsWahab KhaniNo ratings yet
- Chemistry and Digestion of LipidsDocument35 pagesChemistry and Digestion of LipidsBiph BiphNo ratings yet
- Lipid MetabolismDocument52 pagesLipid MetabolismDr.P.NatarajanNo ratings yet
- 6 Lipids Diagestion Absoption NewDocument45 pages6 Lipids Diagestion Absoption Newamel elabassNo ratings yet
- Lipioprotein Lec11 PDFDocument44 pagesLipioprotein Lec11 PDFحسن محمد سعيد جاسمNo ratings yet
- Lipid - Digestion and AbsorptionDocument26 pagesLipid - Digestion and AbsorptionSanreet Randhawa100% (2)
- Lipid Metabolism NotesDocument17 pagesLipid Metabolism NotesLucy ZuluNo ratings yet
- MODULE - FINALS - Biochem - 1Document5 pagesMODULE - FINALS - Biochem - 1Cyril CauilanNo ratings yet
- The Biochemistry of Digestion, Absorption and Detoxification by Prof. Dr. Hedef D. El-YassinDocument64 pagesThe Biochemistry of Digestion, Absorption and Detoxification by Prof. Dr. Hedef D. El-YassinSayhidoen CepexNo ratings yet
- Carol Davila Pathophysiology NotesDocument10 pagesCarol Davila Pathophysiology NotesGiorgos Doukas KaranasiosNo ratings yet
- Biochemistry of Digestive SystemDocument17 pagesBiochemistry of Digestive SystemnishibuchiNo ratings yet
- Review Article: Lipophagy: Connecting Autophagy and Lipid MetabolismDocument13 pagesReview Article: Lipophagy: Connecting Autophagy and Lipid MetabolismPresilyaNo ratings yet
- Integrated Metabolism Lec02Document35 pagesIntegrated Metabolism Lec02Fateha HussainNo ratings yet
- 4 of 7 Metabolism of LipidsDocument84 pages4 of 7 Metabolism of LipidsImad khanNo ratings yet
- Lipid Metabolism NotesDocument17 pagesLipid Metabolism Notessean83% (6)
- LG - Lipid MetabolismDocument116 pagesLG - Lipid MetabolismRawa AyubNo ratings yet
- Lipid Metabolism: Structural LipidsDocument6 pagesLipid Metabolism: Structural LipidsBarinia García LópezNo ratings yet
- Lipid Absorption and Transport UptakeDocument218 pagesLipid Absorption and Transport UptakeSimra Zahid100% (1)
- Lipids NotesDocument4 pagesLipids NoteskatNo ratings yet
- 39 AnswerDocument9 pages39 AnswerJasmine Nicole EnriquezNo ratings yet
- 2.LIPIDS DigestionDocument21 pages2.LIPIDS DigestionAlaa Hisham100% (1)
- 11metabolism of LipidsDocument80 pages11metabolism of LipidsHumrazNo ratings yet
- Energy Production 2. Energy Storage 3. Energy UtilizationDocument14 pagesEnergy Production 2. Energy Storage 3. Energy UtilizationLALITH SAI KNo ratings yet
- BiochemDocument10 pagesBiochemWaseem Abbas ShaikhNo ratings yet
- Hormonal Influence On Lipid MetabolismDocument43 pagesHormonal Influence On Lipid MetabolismKuzhandai VeluNo ratings yet
- Module: 3 Food Lipids Lesson M.III.10Document4 pagesModule: 3 Food Lipids Lesson M.III.10Amit Kr GodaraNo ratings yet
- Compiled MetabolismDocument155 pagesCompiled MetabolismIced WatermelonNo ratings yet
- Lipid Transport & StorageDocument33 pagesLipid Transport & StorageWahidinSchleiden100% (1)
- Metabolism of LipidsDocument96 pagesMetabolism of LipidsLyra GetNo ratings yet
- Unit 8 Digestion and AbsorptionDocument33 pagesUnit 8 Digestion and AbsorptionGlory MuedaNo ratings yet
- (WWW - Indowebster.com) Lipid Transport Amp StorageDocument33 pages(WWW - Indowebster.com) Lipid Transport Amp StorageRs93No ratings yet
- Metabolism of Adipose TissueDocument35 pagesMetabolism of Adipose TissueJustine Joy SolanoNo ratings yet
- Biochem 2.1 Introduction To MetabolismDocument5 pagesBiochem 2.1 Introduction To Metabolismlovelots1234No ratings yet
- Lipid MetabolismDocument21 pagesLipid MetabolismTooba GhummanNo ratings yet
- Lec. 12 Biochemistry IIDocument22 pagesLec. 12 Biochemistry IIGames beautifulNo ratings yet
- Digestion: By: Roselita O. Natividad and Teresita G Montaño, PHD Nat. Science - Ateneo de Zamboanga UniversityDocument5 pagesDigestion: By: Roselita O. Natividad and Teresita G Montaño, PHD Nat. Science - Ateneo de Zamboanga UniversityMini BossNo ratings yet
- LipidsDocument6 pagesLipidsJohn Fritz Gerald BascoNo ratings yet
- 6.1 - DigestionDocument8 pages6.1 - DigestionPraveen KhandelwalNo ratings yet
- TMP D385Document19 pagesTMP D385FrontiersNo ratings yet
- Module 11 Lipids 1Document10 pagesModule 11 Lipids 1cassseeeyyyNo ratings yet
- Discussion Class For Lipid MetabolismDocument36 pagesDiscussion Class For Lipid Metabolismchoices gameplayNo ratings yet
- Biochemistry of Lipids PDFDocument51 pagesBiochemistry of Lipids PDFZayan HaiderNo ratings yet
- Module 8 Metabolic Pathways Final 1Document56 pagesModule 8 Metabolic Pathways Final 1Susana N peNo ratings yet
- Lipid Transport & StorageDocument33 pagesLipid Transport & StorageRaja Friska YulandaNo ratings yet
- Week 6 LipidsDocument59 pagesWeek 6 Lipidsbo aNo ratings yet
- Complex Proteins - Glyco, Lipo, PhosphoDocument19 pagesComplex Proteins - Glyco, Lipo, Phosphodc8mypycmdNo ratings yet
- Komponen Lipid, Katabolisme Asam Lemak, Biosintesis Asam LemakDocument69 pagesKomponen Lipid, Katabolisme Asam Lemak, Biosintesis Asam Lemakalvaedison00No ratings yet
- Lipids Digestion & AbsorptionDocument49 pagesLipids Digestion & AbsorptionMaryNo ratings yet
- Physiotherapy (Post.) Lipid Metabolism DR - Amal BadrDocument35 pagesPhysiotherapy (Post.) Lipid Metabolism DR - Amal BadrthestaffforpediatricptNo ratings yet
- Gluconeogenesis, Glycogen Metabolism, and The Pentose Phosphate PathwayDocument24 pagesGluconeogenesis, Glycogen Metabolism, and The Pentose Phosphate PathwayMelisa Halilovic100% (1)
- Digestion and Absorption of Lipid 12Document36 pagesDigestion and Absorption of Lipid 12Pranjul MishraNo ratings yet
- Metrel Mi 3290 Earth AnalyserDocument4 pagesMetrel Mi 3290 Earth AnalyserMarijan MustačNo ratings yet
- Soiltac Safety Data Sheet: Section 1 - IdentificationDocument9 pagesSoiltac Safety Data Sheet: Section 1 - IdentificationSameh AhmedNo ratings yet
- Master of International HealthDocument5 pagesMaster of International HealthJesper Domincil BayauaNo ratings yet
- Series 70 Overshots: Instruction Manual 1070Document8 pagesSeries 70 Overshots: Instruction Manual 1070Juan Carlos Perilla MartinNo ratings yet
- Tutorial SessionDocument5 pagesTutorial SessionKhánh LinhNo ratings yet
- Volume Booster: YT-300 / YT-310 / YT-305 / YT-315 / YT-320Document1 pageVolume Booster: YT-300 / YT-310 / YT-305 / YT-315 / YT-320SeikatsukaiNo ratings yet
- Connection DesignDocument33 pagesConnection Designjesus curielNo ratings yet
- Chapter 3 - Assessment of PostureDocument31 pagesChapter 3 - Assessment of Posturehis.thunder122No ratings yet
- Anexate IVsolnDocument7 pagesAnexate IVsolnJelena Obrenovic StankovicNo ratings yet
- Sample Cylinders - PGI-CYLDocument8 pagesSample Cylinders - PGI-CYLcalpeeNo ratings yet
- Science Quarter 1 Lesson 10 Day 1Document20 pagesScience Quarter 1 Lesson 10 Day 1Lizbeth Edralinda-martinezNo ratings yet
- 3 D7 QC Physical and Chemical Tests Ok For EmailDocument17 pages3 D7 QC Physical and Chemical Tests Ok For EmailUbaid KhanNo ratings yet
- MOLYKOTE 1000 Paste 71-0218H-01Document2 pagesMOLYKOTE 1000 Paste 71-0218H-01Victor PomboNo ratings yet
- Project PPT Spot WeldingDocument19 pagesProject PPT Spot WeldingMehul BariyaNo ratings yet
- Lab 2 - Capacitive ReactanceDocument4 pagesLab 2 - Capacitive Reactanceali basitNo ratings yet
- RPLB NewDocument22 pagesRPLB NewMeta learnNo ratings yet
- Pengertian Recount Text Kls 9Document26 pagesPengertian Recount Text Kls 9MARINDRA RETNONo ratings yet
- IWGIA Book The Indigenous World 2021 ENGDocument824 pagesIWGIA Book The Indigenous World 2021 ENGREy FOxNo ratings yet
- Physical, Biology and Chemical Environment: Its Effect On Ecology and Human HealthDocument24 pagesPhysical, Biology and Chemical Environment: Its Effect On Ecology and Human Health0395No ratings yet
- Interpretacion Curso Cat CoolantDocument39 pagesInterpretacion Curso Cat Coolanthouston machacaNo ratings yet
- Amp-Eng Anger Management ProfileDocument6 pagesAmp-Eng Anger Management Profileminodora100% (1)
- Method For Emission Spectrometric Analysis of Plain Carbon and Low Alloy Steels Point To Plane Technique (Document8 pagesMethod For Emission Spectrometric Analysis of Plain Carbon and Low Alloy Steels Point To Plane Technique (DiptiNo ratings yet
- Buy Lizol Floor Cleaner 5000 ML Online - GeMDocument4 pagesBuy Lizol Floor Cleaner 5000 ML Online - GeMHimanshu ShuklaNo ratings yet
- Know Your Customer BrochureDocument4 pagesKnow Your Customer BrochureTEL COMENo ratings yet
- EpidemiologyDocument26 pagesEpidemiologymohildasadiaNo ratings yet
- Mayank Yadav: Academic QualificationDocument2 pagesMayank Yadav: Academic QualificationJacob PruittNo ratings yet
- Mechanical Operation Slurry TransportDocument113 pagesMechanical Operation Slurry TransportIsrarulHaqueNo ratings yet
- What Exactly Are Period PantiesDocument2 pagesWhat Exactly Are Period PantiesJay Jay KamgaNo ratings yet
- Mineral Resources, Uses and Impact of MiningDocument4 pagesMineral Resources, Uses and Impact of MiningShakti PandaNo ratings yet
- Microcosmic Orbit Breath Awareness and Meditation Process: For Enhanced Reproductive Health, An Ancient Taoist MeditationDocument1 pageMicrocosmic Orbit Breath Awareness and Meditation Process: For Enhanced Reproductive Health, An Ancient Taoist MeditationSaimon S.M.No ratings yet