Professional Documents
Culture Documents
SEE EC Model QP
Uploaded by
Aarush Pitla0 ratings0% found this document useful (0 votes)
6 views1 page.
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Document.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
6 views1 pageSEE EC Model QP
Uploaded by
Aarush Pitla.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
QP Code: R22
Anurag University
B Tech I Year I-Semester End Examinations
(Common to CSE & ECE)
Course: Engineering Chemistry
Time: 150 Min. Max Marks: 50
Section – A (Short Answer type questions)
Answer all questions (5 x 2 =10Marks)
1. Outline the Beer-Lambert’s law.
2. What is caustic embrittlement in boilers? How do we prevent this problem?
3. Differentiate the primary & secondary batteries.
4. State Anti-Markovnikoff’s rule with an example.
5. Define cloud point and pour point.
Section—B (Essay Answer type questions)
Answer all questions (5 x 8 =40 Marks)
6. A) Discuss the molecular orbital diagram of the N2 molecule and mention its bond
order and magnetic behavior.
OR
B) Describe the various molecular vibrations of IR spectra.
7. A) Explain the Ion exchange process with a neat diagram.
OR
B) A sample of water contains the following dissolved salts per liter. Ca (HCO3)2= 32.4mg,
Mg (HCO3)2=14.6mg, CaCl2=22.2mg, NaCl= 5mg, Silica=2mg and MgSO4=240mg. Calculate
the total, permanent, and temporary hardness of the water.
8. A) Explain the different factors affecting the rate of corrosion.
OR
B) What is a fuel cell? Construct Hydrogen – Oxygen fuel cell and explain with
reactions.
9. A) Discuss the conformational analysis of n-butane.
OR
B) What are substitution reactions? Give mechanism of SN1 and SN2 reactions.
10. A) Write preparation, properties, and applications of PHB and PLA.
OR
B) Give the characteristics of good refractory material. Explain the terms
Refractoriness, RUL, and thermal spalling.
You might also like
- Chemistry 22CYC01Document2 pagesChemistry 22CYC01BonVoyaegeNo ratings yet
- Model Paper 4 ChemistryDocument2 pagesModel Paper 4 Chemistrysazalgola2004No ratings yet
- EN09 104 EnggDocument2 pagesEN09 104 EnggRanjith SomanNo ratings yet
- Btech 1 Sem Engineering Chemistry Ras102 2020Document2 pagesBtech 1 Sem Engineering Chemistry Ras102 2020Hariom SinghNo ratings yet
- First/Second Semester B.E.Degree Examination Engineering ChemistryDocument2 pagesFirst/Second Semester B.E.Degree Examination Engineering ChemistryArshad KhanNo ratings yet
- First/Second Semester B.E.Degree Examination Engineering ChemistryDocument2 pagesFirst/Second Semester B.E.Degree Examination Engineering ChemistryKishore RNo ratings yet
- 18che121 PDFDocument2 pages18che121 PDFRutikNo ratings yet
- First/Second Semester B.E.Degree Examination Engineering ChemistryDocument2 pagesFirst/Second Semester B.E.Degree Examination Engineering ChemistryJyoti KumarNo ratings yet
- 18che121 PDFDocument2 pages18che121 PDFAkash YashNo ratings yet
- First/Second Semester B.E.Degree Examination Engineering ChemistryDocument2 pagesFirst/Second Semester B.E.Degree Examination Engineering ChemistryZander IndiaNo ratings yet
- First/Second Semester B.E.Degree Examination Engineering ChemistryDocument2 pagesFirst/Second Semester B.E.Degree Examination Engineering ChemistryKarthikNo ratings yet
- First/Second Semester B.E.Degree Examination Engineering ChemistryDocument2 pagesFirst/Second Semester B.E.Degree Examination Engineering ChemistryKavya KavyaNo ratings yet
- Chemistry 2023 Question PaperDocument7 pagesChemistry 2023 Question Papercabek22797No ratings yet
- Chemistry Sample Paper 1Document4 pagesChemistry Sample Paper 1Himanshi PrajapatiNo ratings yet
- Anna University of TechnologyDocument4 pagesAnna University of TechnologyakarjunNo ratings yet
- CH 101 PDFDocument3 pagesCH 101 PDFAnsh BharadwajNo ratings yet
- Inorganic Analytical ChemistryDocument5 pagesInorganic Analytical Chemistryapi-37236870% (1)
- Answer All Questions, Each Question Carries 2 Marks: Pages: 2 Reg No.: - NameDocument2 pagesAnswer All Questions, Each Question Carries 2 Marks: Pages: 2 Reg No.: - NameKatrinaNo ratings yet
- Engineering ChemistryDocument9 pagesEngineering ChemistryAnuj EsthapanoseNo ratings yet
- Engg Chemistry R13 Model Question PapersDocument4 pagesEngg Chemistry R13 Model Question PapersBell P PedNo ratings yet
- Btech 1 Sem Chemistry Kas 102 2018 19Document2 pagesBtech 1 Sem Chemistry Kas 102 2018 19Viraj RuhelaNo ratings yet
- Engineering Chemistry 2019 Scheme SyllabusDocument9 pagesEngineering Chemistry 2019 Scheme SyllabusAfsal Sha MNo ratings yet
- Engineering Chemistry KAS102TDocument3 pagesEngineering Chemistry KAS102Tritesh kumarNo ratings yet
- CH201 See Set BDocument1 pageCH201 See Set BJaineel PatelNo ratings yet
- MajorDocument25 pagesMajorloganathanNo ratings yet
- Chemistry 2021 PAPERDocument8 pagesChemistry 2021 PAPERcabek22797No ratings yet
- 21CHE12 22 Engineering Chemistry Model Question Paper 2Document3 pages21CHE12 22 Engineering Chemistry Model Question Paper 2Deeksha V PanchalNo ratings yet
- Term-End Examination June, 2010 Mch-004: Electroanalytical and Other MethodsDocument4 pagesTerm-End Examination June, 2010 Mch-004: Electroanalytical and Other MethodsdebabratasumantaNo ratings yet
- Slow Learner TestDocument2 pagesSlow Learner Testsparkysanthosh69No ratings yet
- Candidates Are Required To Give Their Answers in Their Own Words As Far As Practicable. The Figures in The Margin Indicate Full MarksDocument2 pagesCandidates Are Required To Give Their Answers in Their Own Words As Far As Practicable. The Figures in The Margin Indicate Full MarkssushilNo ratings yet
- CRE-CIA 1-A QUESTION Paper 2023 OddDocument1 pageCRE-CIA 1-A QUESTION Paper 2023 OddA.RAJKUMAR (HICET) HICET STAFFCHEMNo ratings yet
- S3 Main Internal QUESTION PAPER 18 02-2020 EditedDocument2 pagesS3 Main Internal QUESTION PAPER 18 02-2020 EditedACT KeralaNo ratings yet
- CRE-CIA 1-B QUESTION Paper 2023 OddDocument1 pageCRE-CIA 1-B QUESTION Paper 2023 OddA.RAJKUMAR (HICET) HICET STAFFCHEMNo ratings yet
- 9ABS103 Engineering ChemistryDocument4 pages9ABS103 Engineering ChemistrysivabharathamurthyNo ratings yet
- Chemistry 2022 AKTUDocument2 pagesChemistry 2022 AKTUVicky SrivastavaNo ratings yet
- Engineering Chemistry Vtu Model Question Paper 2 2018Document2 pagesEngineering Chemistry Vtu Model Question Paper 2 2018P PrabhuNo ratings yet
- Delta Junior College: OH 4 Aq 2 S 2 S AqDocument3 pagesDelta Junior College: OH 4 Aq 2 S 2 S AqrammNo ratings yet
- Chemistry 20CYC01Document2 pagesChemistry 20CYC01BonVoyaegeNo ratings yet
- 10 - SOT Question Paper End Sem 2012Document2 pages10 - SOT Question Paper End Sem 2012Harsh ThakurNo ratings yet
- AtomicDocument2 pagesAtomicAnnu yadavNo ratings yet
- Engg. Metallurgy M Iat - 1 Question Paper - Set-3Document2 pagesEngg. Metallurgy M Iat - 1 Question Paper - Set-3SCT HOD - MechanicalNo ratings yet
- WWW - Manaresults.Co - In: I B. Tech Ii Semester Regular/Supplementary Examinations, April/May - 2018 Applied ChemistryDocument4 pagesWWW - Manaresults.Co - In: I B. Tech Ii Semester Regular/Supplementary Examinations, April/May - 2018 Applied ChemistryMunawar MirzaNo ratings yet
- B.Sc. Dual Degree/M.Sc. EXAMINATION, 2022: Time: 2 Hours)Document2 pagesB.Sc. Dual Degree/M.Sc. EXAMINATION, 2022: Time: 2 Hours)Vishal TanwarNo ratings yet
- Chemistry IMP 1 PDFDocument4 pagesChemistry IMP 1 PDFDevanshi PatelNo ratings yet
- DCH109 (E)Document2 pagesDCH109 (E)Vishal TanwarNo ratings yet
- Sure Shot 6Document27 pagesSure Shot 6abiNo ratings yet
- Chemistry Board Papers 2006-2017 PDFDocument227 pagesChemistry Board Papers 2006-2017 PDFAgape Sol'ns100% (1)
- ChemistryDocument2 pagesChemistrysushilNo ratings yet
- Term Test For GM1 and BM1 Chemistry ch1,2,7,8,9Document3 pagesTerm Test For GM1 and BM1 Chemistry ch1,2,7,8,9Rana Hassan TariqNo ratings yet
- Chemistry Model PaperDocument2 pagesChemistry Model Papershop63pNo ratings yet
- TS JR (Pre-Final-2) (Chemstry Q P) Ex DT 17-04-2021Document2 pagesTS JR (Pre-Final-2) (Chemstry Q P) Ex DT 17-04-2021AbhiNo ratings yet
- BSC I Year - Chemistry Paper-I - 2015Document3 pagesBSC I Year - Chemistry Paper-I - 2015Urvi KaleNo ratings yet
- Paper Vii Model 2Document11 pagesPaper Vii Model 2Monica SrinivasanNo ratings yet
- Tenth Class Physical Science Model PaperDocument4 pagesTenth Class Physical Science Model Paperkatta swathiNo ratings yet
- CBSE Sample Paper Class 12 Chemistry Set 1Document4 pagesCBSE Sample Paper Class 12 Chemistry Set 1NeerajNo ratings yet
- 8630ENGINEERING CHEMISTRY Model PaperDocument2 pages8630ENGINEERING CHEMISTRY Model PaperkasimalaniharikaNo ratings yet
- Engineering Chemistry - II: Code No: CY16121Document1 pageEngineering Chemistry - II: Code No: CY16121Kishore NagaramNo ratings yet
- Anna University (University Departments) : Roll NoDocument2 pagesAnna University (University Departments) : Roll NoArvind SriramNo ratings yet
- 1st PUC Chemistry 2014Document2 pages1st PUC Chemistry 2014sathishNo ratings yet
- Certificate of Completion: Kotakonda Jairaj MaharishiDocument2 pagesCertificate of Completion: Kotakonda Jairaj MaharishiAarush PitlaNo ratings yet
- Bee Unit-4Document49 pagesBee Unit-4Aarush PitlaNo ratings yet
- TransformersDocument23 pagesTransformersAarush PitlaNo ratings yet
- Aa Rùocks/Kaa Aa Rùocks/Kaa Aa Rùocks/Kaa Aa Rùocks/Kaa Aa Rùocks/KaaDocument7 pagesAa Rùocks/Kaa Aa Rùocks/Kaa Aa Rùocks/Kaa Aa Rùocks/Kaa Aa Rùocks/KaaAarush PitlaNo ratings yet
- Assignment IIDocument1 pageAssignment IIAarush PitlaNo ratings yet
- BEE Important QuestionsDocument2 pagesBEE Important QuestionsAarush PitlaNo ratings yet
- Microbot 10x6 New Mini DroneDocument1 pageMicrobot 10x6 New Mini DroneAarush PitlaNo ratings yet
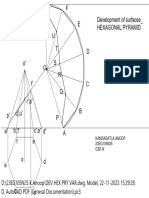
- DEV HEX PRY VAR-ModelDocument1 pageDEV HEX PRY VAR-ModelAarush PitlaNo ratings yet
- I-B.tech-Ii-Semester Time Table 18.01.2024Document17 pagesI-B.tech-Ii-Semester Time Table 18.01.2024Aarush PitlaNo ratings yet
- VishnuPurana 10024433 PDFDocument348 pagesVishnuPurana 10024433 PDFSailesh HanumanthulaNo ratings yet