Professional Documents
Culture Documents
Chemistry Hand Written IPE MIMP Qestions
Uploaded by
angadibalajithkumar0 ratings0% found this document useful (0 votes)
154 views58 pagesfor eductional purpose only
Original Title
chemistry hand written IPE MIMP qestions
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentfor eductional purpose only
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
154 views58 pagesChemistry Hand Written IPE MIMP Qestions
Uploaded by
angadibalajithkumarfor eductional purpose only
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 58
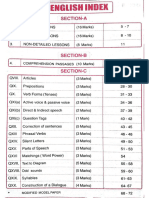
[sel alias saclay: Wo Fao
1 | orice shsucture. aie \-
2 |closification of | 3. & aot
ERements
3 | cherscal Bonding 6-% eS Pe
, a
SAQ's
te | States of matter I-k 13-18
5 Thermodynamics C+ 1q- 2h
6 | Organic Chemistry 8— 12 22-25
+ Cherrical Bending 13-1F 26-28
8 |ist Qnoup 18-20 29-30
1 |Hydvogen And Hs | e124 Bl- 3y
Corrrpounds
'0 |Stochiomeby [24728 Sus ss
ul Chernica) equidibyd 29-33 Bqa ue
Km Aids and
bases
S-8l chapker Name ished: O-o Fg:No
12 | States of Malter I-6 | eg
13 | Stochiomedtn zo t be
1 ' ane
ae men Mirae
iy Ovganic chemistry 13-23 | tae
te | Eavironmental chery Ru 33 ome
tt | (uh quopp Elements [5-4 PO
18 | DATA Gowup Elements [5-55 | 51-53
Chemical Bonding i 66- 60 53
LAG if
\What are the postulates of Bobs made! of Fydragen |
atone? Discuss the Importance of this racde! to expbin
\avious sevies of the Line spectya ?n hydyvagen atom?
Rostulates:- eo
) The election in hydyogen atom veuolves around the
nucleus fn.a ftxed civeulay Patith called ovbrts
2) Each orbtt ts Associated with definite encigy-Sovhed
ave dalled energy leuels - i
) “The energy levels ave repvesonted a 1,253) ~~~ Can) kyLyrafh
form-the Side of Nucleus:
H) The angulov moment ofan electron ts al
moulltple of ft . hey
cays Freya)
lalhevey rn= mass of electron
Ve ueloctty of electron
v= Radius of oybrt
N= prtreteal Quantum
Nurabe
he Planks Constant rs
leuel tf may e@ratt Cov) Obcorh enewy
te,
vuhere; x= Energy of higher ovbtt
Et= Energy of Locoay evbtt
HYPROGE NS SPECTROM: ~
Ee} tay
Urs [4 ty] 16
Ace) Quantum numbers ave lo exphin complete explinatton|
lalhove, Ri= Rudbera's constant = 1)09-6330m"
m= Locoey orbrt
92= Highe vorbHt
OFAGRAM:- Chae
ST ee orien
=b
we - | [Pind Sertes (far ae)
Evy |, Ul] Bracket sevtes Contd te Tm)
; 4 | IIIT pashan sertes Crear re)
aa LIT elamer sortes Cuter bie veafon)
nef Lumen Sevies Cuy vegfon)
Nawne of Sovies Region nm ng
Pia Br. meee
2) Blarmey utsfole 2 144
3) Pashcor neavdR teens
4) Bracket middle ae 1, a
5) Hund a
fav oe 5 6)9)----
How ate guardur numbers Pid and ry awited at?
ExPotn the signtftance of these Quantum numbers
Lov) Address of electron finan erbrt “the -four Quarrte|
Nambors ave :-
( Principal Quanturn tum bey
(fi) ARmuthal Quantum Num bey
Gi) mMagnelic Quantum Number
(i Sptn Quarrlum Number
O38
{o Principal Qeanturn Numbev Cn): — 21>
DS eas proposed by Mefls bhov
2) D4 ts denoted by ‘o!
By the ualues ave 0% 3) 4 ---- Cov) Ky Li D1 N- ~~~
u) the maxfum number of electron fov gtuen ualue
n=on”
h
6) Angulay mementum { muy= er
Significance: Tt tadteates stze ofan orbt! and energy
ofan electron:
Gi) Aztmuthal @vontum Norobev C/):-
NE ceas proposed by Sommey ferld .
DL coos doroted by Lotter \y!
2) When x Ualues = oy 27% ----C01)
4) Argulav Mormertuny =f Jd)
Signiftconce- Dl tndtvades the © hape of ovbt{a |
Orbital Yualue shupe
Ns ° sphovita|
a 1 Durnpte
ad 2, Double pumb_bep
“EF z
Fouv Fold dumb -bet)
(@) Magnetic Quomturn Murnbenr-
Lt coas proposed by Lande
2)94 is denoted by WW
3) ualues ae =-hoy tl
u)Tolal Ualues = 214)
Oy
Signifinance Tt todscated the ovientatton of ovbrla!
(tw Seta Quarlum Murnben:-(s)
) St cans proposed by Goudsmith and ublen becly
2)P} ts denoted by 3!
3) °s' ualues ave tand =f
4TE the Clectron veuolves around Clocle cotse Cs) dtvetion
Spin fs +1 and fFelechen veuohes around antt-clods
colsec\) spin ts ~b
Signffrancer T4 tnditcates the divec-ton of lhe stn of
-the electven-
ic
©] Deftne Tec and T& + tohy fs Ler 7E, fora gfuen ata,
Discuss the factors that effect Tt of an olectyan
DP Dootsatfor Lerthaley Cov Polertfal Cre!
The chewy % vequtved to rermove an elecdvon frorna
Neutval ; fsolated gaseous Q4orn 1s Called Te;
4 Met TE 9 witgy + 1
2 Fovisatten Erlbalpys- (142)
In)
“The energy vequtved +o vernate an elector fron,
unt postttve gasceus fon Called TE,
+ 40
1)-+ Tp ~—> 79) + ©
ra
oS
that Is a pevedte property? Uoco-the Follocsing praperttes
Ua ina group fna PerodfC ? Explain:
@ Ptomee vadrus @ Election gata eottalphy oven
ore @ Natuve of ortdes
CEN
Poredic Propertes:
The vepetttton of properties of elements of veoulay
fntevvals of 4tme fs alied PeIedtetty and the prepewel
Ave called pevodte Properties:
@) Atomic vadtus:-
The difsfance helwaen the Corby of Nucl and athe
Cutamest shell ofanatom ¢s alled AR.
GRovp PERTOD
Ina Gap fie top-to Th © pevfod From lat ty
Bollore AR frcvers) . VOHM+ ComPe radfus ec reas
Becauk dt fle vertattrs Because Ath fovensert 5
enters Thto need ghel! ity ©
enkers to40 samme shel} -
CB Lonizattsn Enthalpys ee as
The mfnfurn amount of eCnerg 4 ¥RQUP LOD 10 Vernove
the cutev moc+ & From Qarour nou tral atom catia
te
Moy + TE, >My) +e
06
(3) te, rteu- Lee =I
Second Tonfsa-tton enthalphy fs alesays gvea tev thon
the ftrsd Tonteatfon emthalphy Because move effecttve
nucleay Chayae'
H) factors tnfluenetag tonasation emthalphy —1,r4
@) Axtomic_vadtus: ~
As the Plomie radfus fncveases;-the Mucleav fevee of
Adltyaction guer the ualency Cledvon clecrarses.
(® Necleay charge +-
Tonfsattor erthala Increases ff fs Because the
force of attraction on tre ualere Clectron trcrecos
Nucleay chavge y TE
© Scveentno effect :- *
T+ veduce’ the fowe cf AH ractlor Joucavds Nutleys
Here outev mest eC lectyor can be easly removed-
UigheY the senting affecd “The Lesv-+ho UalUo of te
!
Screening effect y ae
(@) Penetration of orbitals of yalenve Clee-tvons :-
More Ff the peretvatfoo of ov bfta) move etl be
the fonisattén enthalpy
"the Order of penetration Prewoy its [srp>aof]
: J
ot
GrRoop
Tnagroup from top +0 Bottom Te decreases Recause
92 Fncveass *
PeRSoD:.
Sna perFod frarn Left to ¥fght Te Therea®> Because
PR decrana
Coy ELECTRO MeGpTTuTTY Cen) —9m
the ve latte tendency of an olor -to attract He
Trared pate o¢ Ptocerndss Plsott & ated ev:
GrRoup
Th aprgrouP From top to bottom EN decrarss Boxe
Otto. vadFes Ther eases - .
PERSco!
Bn a pertod from Left to vight EN Increase because
artoenic vadiue clecren ses
WD EA CEleckon AHtnity)
Len cine ef eneqy welecsed cohen an election fs
Added t> the valency shell of nevlol qaser abasic :
called election ostfini ty
C eo, AEA
Algu& Bi
Groupe
Th oa qreup from top —bettery A clereaces Ihe cause
orttornic —vadiug — Incre aces:
oe
Pentecd-
dn a pertod -from lett to wight EA teevenset because
Atoen'c vadiuy cecrenses-
© nlatuve of opiden-
the elements when hated seth Ongar -forms — cowesponding
odes:
srTRese axe 3 types: Dacki'e a) haste ayammphoker’e oxides
Group Period
D Beidic orlces Clecrenses Increases,
©) Baste oxfdes seairennses decvates
LibHe on essay on Sifds¢ block @ lements.
(eer
Depending on the eleélons ave classified on tu Hocks
a) S-bleck elements. an
then de-frerentiatig election enters into orbital is s-block
athe General electronic configurdlion ts ns'>
D Tt contoins a-qreups ies) BA -ns! ITA -nc®
Peblock’ etennentit.
the dletperentioting elertion eritey int poBlbak ye
pr block etements
an
athe General electronic configuration ts ns opt
grt wo placed vight — sicle of Periodic table
wT contains 6 qroups
cS
9 O- Block elements; —s 2m
) When differentiating electron entevs into
Ch-i) d-ov6ital jg called df-block efement s
The qeneval € fectronic Con fiqara tion 7s (n-)
id '7!0 pF 5101) > “
2) Jt was placed in middle of 5 & p- block Elementy.
4) The Qroutps ave cesiqnated -from 3-12.
id) E> Block € fements.- eae
) when oitfferentiating electron enters into
(n-d f-orbital 78 cafted f-block Elements.
P)The geneval electronic config wratton i$ (n-2) ¢'
en=1) doting
3) These ave placed in bottom of the periodic
table.
4 4t contains Lanthanotdes § Antinides.
What do you Under stand by fy bridi sation?
€iplain dtfferent types of Ay bridisation
involwing séporébital.
Hy brief iat
[The intermining of atomic orbitals faving same
Enevqy to produce Equal no-of Telentical fy bride
ovgitals 1S Called Hybridi ration
— 1M,
: ®®
f ; s
D) sphybridisation;- —>.M
the inter mixing ofone's'and one P orbitals to
Produce 2 SP Aybride orbitals is called s-p
hybridisation.
+ S/.=S507.and P+/-=S07-
I> Shame 7s linear and Bondangle 5 180°
O+00 ——s
$ P Sp-hybride orbitals .
Sxample,-
Bech molecule:
4
DJn Becia, Centre atom Be’ undergo's p’ Ay brichisatigh
b) Shame 75 fineay and Bond angle (80°
3) In Bech Contains 2 «¢, Bonds.
cl — Bet cl
2) Sp? hy bridisa tion ;~ —52M
The intermixing of ones‘and 2 p-orbitals to
Produce 3spr hy bride orbitals 7s called sp» ~
HG bridisation
[> S character = 33-334.
r> P Chavactty = 66: 66+
> SRape of the molecule 7s tigona ¢ plannay and|
Bond angle iS 120°
fi
L
+—
O ©@®
O+2 = sa
Ss
3-Sp2 hybride orbitals .
\
Example;-
Belgmolecule ;-
D $n Belg ,Centralatom ‘B’ undergoes spt hybridis-
lation.
K i
It contains 3 ora bonis.
3) Shape is trigonal planar and Bondangle is 120°
chy
eur T
3 sp hybridisation~ —s 944
The intermixing of one's’ ¢ ap-orbitals to produce
MSP3 hybride orbitals is Called sp3 hybridisation
[> S-chavactey =254.
[> p- chavactey - 457
le Shape is tttrahedral and Bond angle i's (09°28)
ee
$s
U -Sp3 hy bride - 016i tal:
Srarmple:- P3 hybride 7 S.
CHu mote cule;
D9n CHy molecule central atom’ undergoes sp3
(>
7
Hybridisation
@ ®
b) Jt contains Y ogps gbonds
3) Shape 75 tttvahedvaland
4) Bond angle fs (09°28).
-
Give-an account of VESPER theoryand TES
applications?
Pos tulates;-
) The Shape of the molecule clepends vPOn
number of Electrons pairs in valency Shelf of
Centralat'on.
2) The shape and bond angle depends on repuls fon
Between electron Pairs.
2) lone paty of Electron occupies more Space the
bond paty of Electrons.
W) The vepulsion order Of Electron pairs .
S) Repulsfon among the various bond
No-oF eLlectogsNoof [No-oF
Pats "eon pat tone, | Shape [Bond Rs
; paiys angle oe
7 2 > flineay | 180° [eect]
®) = ° ITvigonal
u Plannay| !20° | Bel
«RGM 9 etrahedat!09°2' | CHYy
_8®©_
ml 3 \ Pyramid toe? | NHS
ie bs 2 gular] toy? 26
= Si
© frigonas | go} 20° | pets
foi Purana
6 6 ~aot /
0 octahe aorieot SF6.
aieiee
natts molecule: - 32M,
=
2D In Nig Molecule ‘N’ ator undergo Sp3-hy bridisatig
) It forms 3 G3-5 bonds .
B) Jt Contains Bond patys=3 .
done patis=;
4) The vepulsion s of B:Pand Z:P Shape 7s Pyramidal
and bond angle is lo#®
“aS
hs bes
Is
Given the moleculavoreital energy diqrarm of
Naand 05 Caliculate the vespective bond ordeg
=
)N2 contain [U electrons
2D Bond order = Nb-Na _
By
= [Oeu. |
“> = 62=3 CNEN)
3) gt ts diamaqneticnatune 1m.
Au
d —> 2M
Thapy T2P2
[1
hy ri
2s rb
Fl
o2s
iL
x
hy
| Ve* 1 ee Ise
ols: |
(Oar \
pdn or contain 16 Electrons. im
2 Bond order = Nb-Na_ lo
a a ale,
BO
fo
3) Tt
fs Pararnagnetic fatue —> i
ra
2S)
as;
SHORT ANSHER QUESTIONS
STATES OF MATTER
Write the postulates of Kinetic molecular
theory of gases:
() Gases consist of large number of tiny porticles
called as molecules -
(2) Gas molecules move randomly tn all dtrections
with different Velocitres-
(3) Collisions of qas molecules ave perfectly elastic.
(4) The actual volume of the gas molecules t's
negligible In comparision to the total volume
af the container:
ts) Average kinetic energy of gas molecules ts
divectty proportional to the absolute temperature
State and explatn Graham's law of diffusion.
At constant temperature and. pressure the
yote of ali-ffusion of a given mass of a qos
is Inversely proportional to the square root
of its density:
y= Yate of diffusion
d= density af a gas
Med
t
Vet Ta
3)
M= 2x Vel
neve Im _ Ra
% Ja IM IVa,
Tf tt ave times taken -for -the cli-ffusion
of vy, ave volumes of two different gases
then rates of diffusion is qiven by
Nite
vt,
a
te
Applications of diffusion :
1) Molay mass of unknown qos ts determined.
2) Tt ts used as Ansils alarm,
Decuce (a) Boyle’s law (by Charle’s law
()Graham’s law (d) Dalton’s law ce) KE from
kinette gas equation
(a) Boyle’s law:
According to kinetic gas equation
Pye. nmonu>
Pve = -Lrenv'—_@
Kk E=tmnvt—G@_ | Pvetmned,
According to kinetic molecular | Py=Constant
theo kELT |
KET
KE =«-T —© (k is Constant)
| From equations O,@ £@
i!
|| a
Ve Ek
| Pp ZK
Pv= Constant [At constant +]
”, This is Boyle's law.
w Chale’ s low?
From Kinetic gas equation
A .
Py=temnu Formula’
i
Pye = bmn Pv=t-mnuyins
Pye 2 ke veh. kT
3 sz P
——
Pua 2 eT KEaT
ve 2. kT KE = tmnt
KI Lmnu'
|
At constant pressure veConstant tT
VAT
« This is Charte’s lav.
() Graham’s laos
Py= + mnv*
| At constant pressure
ails
Urns * TE
[ee] [? vores J
“. This is Graham's low of diffusion
(4) Dalton’s Law:
Consider ‘n’ molecules of a gas ts present in
a vessel of Volume'v’ and m, *s mass of gas
molecule and v4, IS ReMes Speed .
Accorcling to kinette gas equation
2
= mny, et iss
Ray m™e —_@ Pv=-bmnuvns
Ty the gas is replaced by another qas
taken then :
Py ap Te —@
Suppose the two gases are taken together
in the Same vessel: let the total pressure of
the mixture be Ppp, then
Pete 7 rou Lo mn, uy
ol 2 aot s 5
vi Vv
= P4+P.
Total fe
Ce) Kinetre energy from Kinetic qes equation +
According to Kinetic gas equation
Py=tmanu"
Py = = srn™
Pra Ek—@ Formulats
- NaS ,
where & is Kkinette energy von
&.= pv Ee pe
az
&= 3 et E.= 3 ner
6, ~ Smet (For 1 mole of ges Pvener]
State ancl explain Dalton’s law of partial
pressure |
At constant temperature and Volume the
total pressure of non-reacting gases is
equal to Sum of porttal pressures of all qeses
Pee ee ee (At Constant v7)
where 2,P,R -- are partial pressures of
Gases
Accoreing to Tdeal gas equation —, 1m
Alccoreling to Dalton’s law
Pa A+R +---
Pa RT, ART, MRT
Vv
o— PM
Mole fraction % Total
pressure
Gm)
THERMODYNAMICS
State and explain fisst law of thermodynamics
Explain - :
First low ot thermodynamics :.
STKis is brown as “fow of consesvation
He con be defined in morn, ways:
8 Enexqy con neither be created nov be destroyed:
But it con be converted from one fosm to
ancthex form: —> am
of energy
Let us assume that o system * A" having intemal
enesqy Ua absoshs 0 cextaln amount of heat
eMeQY CQ) from suvsoundings and undego a
charge in its stole to Big Ug.
tH) tet the internal enemy in the state B is Up -
tet wW amount of wosk done by system -
ty The net gain of enesqy (Q-w) must be egual
ito chonge ‘nm internal erexqy (Au) from Fisst law.
4u> (Ug-va)
Q-w
()
Q= AUtW
For infinite simally small changes
—> OM
20
Stote and explain Hess tow of constant heot
Suramoation
Hess low of constant heat eummation states that
total heat charge tm O veaction is “the same
wheathes the ehemical seaction takes place in the
One Step (or) im the ceveval steps —> am
ST path Bs" A’ changes to By disectty in one step’
A—>D AH=4+Q Aer
b %
Br path Te bad
A changes to D in 3 steps ° 8 dy.
A—> Btd, Cov) AH=-2,
B—> cg, (or) AH=-% >
¢ —> Dtd3 (ors AH=-2y
The total teat chonge In the path Q
Q= 9,4 Wt Fa — IM
ex CO, is obtained rom (Coraphitdyand Os in rrany
ways
step- Bt C40,
ta Os ¢q) 3» OH=-393- Sk FE
step-Ir + (?) C#AL Os (gy > Com) > OH ~lto-S kT
ud Cott Oy —s COs.) OH=~883' 08 RE
Total Av= (-1to-s)+ (assoa) = ~3¢3-sakT
1M
+t
Define Heat capacity. What ose Cp and Cy 2
show that Cp—Cy=R -
op is Molex heat capacity ot conctont pressure
Cy is molox heat capacity at constant volume.
> IM.
Heat capacity of a substance Ce): -
Tris ts defined os the amount of heat reguived
to wise ite temperature Hough a —> IM
a ae Y= heat absorbed
{a= du+pdv] ae esise in tempenatere
we com write eguations ag
At constant pressure :- 9p = CpAT =AH->@
constont volume :- MW, = CyAT =AU-3@
‘From Tdeat qas equation
Aut ART =AU+RaT
SH= AU+ RATS @O
— IM
Put OF @ m @ we get
CpAT= CyAT+ RAT
Cpat = AT (CytR)
Cp =Cy+R
At
gr
ORGANIC CHEMISTRY
Complete the following veaction and name the
product A,B and
cae, a0. A Hot metal, Q _Alel 4
tube CHacl
| Coc, BHO s copy Hetmetal Cott, Als Core city.
tube cH cl
A —> OM (Acetylene)
B —> CoHe (Benzene)
C —— > CeHs CH (Methyl benzene) fum]
® Nome the product 4,8,¢ bevmed in the follow
seaction ¢ °
Ethylene Brs /ecly, A Al se Bh Lo
ee re (\,a-dibromo ethane)
BY RY
B—s cHecH (Acctylene) -
7s By
| ¢-—~ he ch (a1, 2,2 tetra. bromo ethane) Cum)
1 By Br
© Give two examples each for position and
CHonal teomesiem 9
The compounds which axe having same woleculay
fosmula but differ in thely position of atom (or)
functional group ts called position isomerism:
23
Faw Curae CuHge |
HoH WOW
H 4 em
pop d—b Bie wah bdo
a Ra) tha Moet mw oH
t-chioro Butane
Eat Catgd Cute 0
H H ow H
Be) EU) bey eet
Ae te eee H-E—e— b-d-4
A Aa HOH oH H
t-Butanol &-Butono|
¥) Rurctignal Tromevism -
[The compounds having the same moleculas formula
but different functional
qmups
£9 dy Cattg0 CaHg0
Hou oH How i
-b-b-b-on H -t-b-o-b-H
ey Ale and
tty Cys 0s CyHg0,
wow OH M liot |) a se
H-t~ d- d-coon H-b- ob bey
& |, 3 a tot
HoH HOH
+
What do you understand about geometrical
Esomerism: Explain geometrical tsomey of a-butene
FExomess hoving some structural formula But
differ in the spatial asva:
ement of the
an
qmups sound the double bond ave called
geometrical isomer *
There ave two types
hy) cis -isomey 8) Teans~tsomex —> aM
) cis isomes: tuhen the Same qsoup axe tie on the
some side of double bond then it is cig—icomer-
Aas un
c A Ze 8
c=c
i Nu
H
cis. tsomex oe UM
8) Trans - isomers: then the some group ase lie on
opposite side of double bond
cH a
uf
Hany | Peete
Trans—isomey => 1M.
Explain the method of writing €-% configuration
for geometrical tsomersa taking cHct=CFBy- as
example -
Ge Ocguntene
=~ configuyation: when atoms have higher atomic
Number axe on same side of double bond.
[Tt ig BZ configuration - =I mM]
c Tig
w F
Z- configurati on)
Ldhen atorns Of higher
E- confiqurakion:-
are om opposite stde of double bond
E ~ conf tg wxation
cl Fe
Sea
a
—E-c on figuration
aM
atomic qumbbey
it hove
— IM
26
CHEMICAL BONDING
molecule 2
Explain the hybyidiga tfon fnvolved tr Pele
Ans) Pa Pets Molecule ‘P? te the central arto -
— I
% Shape is tHgona| (Pere) bi pyromicial ect
3) Bond angles faye tage Wea 120" : peed
&) Hybricisailion — fs sped ae
4) Pie + bonds are present eS ar
\[Explat the hybridization involved 0 SF6 rdlecule?
||
Pn She olecule ‘S is the cental ator ny
2) shape ts octahedral = 1m ee
3) Bord angles are a0 ard tee" RSgHF
«) dybridtcation te spd pF
5) Six ‘so ' bonds are present {oe tabedvat)
IM
@ State Fajan's Rules ond give sutable examples 7
Ans! Fajen vles explain the partial covalent chonacler
of the Tonite bonds
) Por a Qiven cation covalent character trereaser with
increase in stze of anfon
Qi KT ts noe covalent than KF —™m
2) Covalent character fneveases with Frereasing charges
of etther cation (61) anion:
Eg Se Clu fis more covalent than Sn ly LES
—_—
there are -two types of hydrogen bonding
DDriter Molecular hychogen bending 2. The hylrogen
bond formed belween cirfferent polar enole culee
called ‘inter molecular hydro ger bonding.
roe. H-F
ee caer he)
4 — IM
i) Tota molecular bydregin bending:- the hydrogen
bond -formed pelweer two atom of same molecule
iS called frita moleeulay hyhogen bonding -
ae oo
eye ok
—™
2» For a que anton covalent character increases utth
decrease tn size of cation
qr Lip is enoe covalent fs higher than kF Be
W) Covalent character is higher -for compounds cations
with —Reeudo inevt Gas con figuration than with
inert qos configura tion .
Gx Cucl ts More covalent than Nall sim
Define dipole moment? write fs Appitcations-
Beef fnttton:-
“the produet of magnitude of charge on the
poles ard the cléslance belie two poles i the
tipole enoment- aM
Tt % denoted
Applications:
) be delermined
D Geometiy of the molecules can
2) Cre ard trans fsomers con be separated:
3) Bond angle can be cetermined
WD th of fonte character « poke 2100 —— 1m
———_—
What is hydrogen bond 2 Explain the different
of hydrogen bonds with ae fferent types
The weak electrostatic doe of attraction between
partially - postttvely charge hydrogen actom ond
highly electionegative atoms (FON) fs known as
hydrogen bond 3am
a
13°" Group
Explain the stwuctise of Di borane:
Ans Shuctine of Diborane «-
Di borane ts an election deficient enolecule
i 4 tof
h-¢C - ¢-A H-B - B-H
1 \ i :
4 H u 4 — |»
Orbital structive EF Diboranes-
DT APhorane each boven atom undergoes sp?
hy bral? sation.
ld) Diborane contain 4 terminal
2 bridged hyckogen atorns
In Atberane bonded
hydrogen ates and
5 (| Besant ieatoane +o 4 terminal
Hychogens Hough sp" § bends
t) B-W-B bend 16 formed bby the overlapping oF
Sp hybrid orbital with ere electron -from one
own atorn sts orbttal of hydrogen actorn ond vacant
ép hybriel oxbttal of onothev
a-three contred +? elector bond
banana lord ©) tau
AC A
wn gO" i aa, Siete
a’ Qa “kK w ue
gs known as
loond- 32M
ay
Aes)
®)
| Expl borax bead test wlth 0 suitable example |
On heating hoor first Loses uaciter—rnoleculer and
Swell: up on -further heating TL teans toto oO
-Lrang perent Ug utd — wohich nodes toto glass rratevia)
know as borax bead - a
A
No, ByD. tomo > Na, Bud, —> 2Na BOr+ 8.05
Usedium metal Corte anhydride)
bovate 1M
For example. lhen boar % healtd to bunsun burner
Flame with cenbalt oxide on a loop of pleitiques
volte 5 a blue coldured C0 (B0:): bead is
B:03 +C00 — C0(R0,), 1h
Give two cnethods Of preparation of Atborore?
Reparation of de bevare (BH. s-
Bets rack with idly to form drborone in
Preserce of dle thy! ether 2M
UBcls +3UBlHy 328) Hed aur Cl +38,
DBR veact with Nat -to form — diborane
apratenah 4S Buy +6NOF mtn
3)
HYDROGEN AND LIS COMPQULDS
PENS ISS, Goacfeal NonaON An “ah Sek WO, CON AEN
198 eafleRafing a3 Loall oa xedudiog, agpete |
WP CAS Og baty osldiaing ond xoducting ongrks
CAESA Yagattas:
(OMe cases Wack toad suithe to with land s.igeate
PPSUS*IN,O, —SPosoughADER SM
(
|
DX o*e 238 |
SRS aRAifod Looms Slrake Ao honge. Saignete |
& *
| Brae, MS ocean rae |
Reduding WoPEdfoe-
Nypochlorous acid is reduced to cl” ton
HOC] + HrO2 —yH30*+CI7 +09 = =—s ym
| {
| (2) Im presence oF base I iS reduced to Iodide (r~fon) |
T2+ Ho02+ 20H ——y2T + 2H20+02 —> IM: |
|
| ; ; \
22) Explain the Following with suitable examples |
| Wy electron deficient 1
Ui, electron precise and
| ali, Electron vieh hydrides
|
32
Ans
23)
When dihydrogen react with p+ block elements Forms
molecutay hydrides:
dy Electron deficient hydrides :-
These hydrides hove the lesser Dumber OF electron to
write theiy lewis structures
£q: Diborane (Bs Hs) =r
diy Electron precise com, ounds +
“These hydrites have the required nureber of electrons
to write ‘theiv leis structuye
eq: CHy » OB a =~ et
Explain the..terms hard water and sort voatey , corite
a note on it:
i, Calgon method For the removal oF haydness oF water:
4, Soft water? water which gives good quantity oF
lather with Soap Is soft water: —> 1M:
2 Hard water: water which does not give good
quantity oF lathey with soap is Known as Haid water
3, Hardness oF watey is due to presence oF dissolved
Salts oF calcium, magnesium as bicarbonates-
4) Hardness can be removed by calgon method
and lon exchange method
ame Sh
it var Oi
| cal gon method : sodium hexameta phosphate (Nas Ps O12),
aw e> i
7 ey
Lis known as calgon:
| ahen hard water is passed ovey calgon calLium and
' Magnesium cations are removed as Follows
| NaGPe0is —> 2Na*+ [ay Pe Ore)”
cat® + [Nay PeOw]”> —s [Nas Ca PeOwe) “honor |
721M:
2u) wlrie a Few lines on the otility oF hydrogen Os
| a Fuel
A‘) 1} Pollutants in combustion of hydrogen witl be tess thai
| petrol
nD
IM
;2) Atomic hydrogen and oxy hydrogen torches ave
used for welding and cutting the metals |
— iM
,3) Hydrogen also used as rocket Fye} 3M
4
4) Hydrogen also used to Fuel cells For gene rating
electrical energy -
STOICHIOMETRY
Balance the Following redox reaction by ion - electron
|
jMethod taking place fn acidic medium:
@ (ro¥ 4 Noy ——» Cry Nog
(by) Mnog + $03" —_, Mot? 4 sou? P
(a) cvs OF? + Nod ——> Cr#? 4+ N05 ¢-
oxidation
a a
Creoy + Noy —> Cv*>4 Nos 3 1M
NS Ain at]
Reduction:
dyidation haf reaction Reduction half veaction
) Noy —» Noe 0) creoy? —> ¢r#3
- a>
nitrogen atoms cre balanced (¥g09" ——» 20
ev are balanced
: — Nos e
po) eae stb 0 J) Cys 03’ —> 2¢1* 94 4 Hao
oxygens are balanced oxygens are balanced -AM
la) wos + Ho — Atos + 2H* (3) Cry07°4 1YHt—y 2004 H20
Hydrogens are balanced Hydrogens are balanced
- eo -. 43
[U) nloy +H2.0—> Nogtent+ 20° —O lay crpog ets 6 Der + # tao
—-®
changes are balanced — 4M
charges are balanced
i
i
Ox 3N0+3H20 —y anos +bH t+ GeO
Oxi > Crrog4tynt 46e —> 2144 FHr0
x 2 +3, g3+t4tHro
4 @ Cr20743NOT 4 RHI —y BON + SN03 ie
adh
25
*) Given oxidation
: se we
Mnoy + $03 —» Mnt* 4 sod
RSE! ERD LAE
reduction
Cee ae St 0) Moog ——+ mp%* roll
sakoms ane balanced fais vel Ldotanceds
nee ste > sol? @) Mnog —— Mn*? + a Heo
oxygen atoms axe balanced —— prugen atoms are balanced: 1M
(@) sob"4 Hao —> Soy "+ oH @) Mnoy + 8Ht —> Mn+ GH20
Hydrogen atoms are balanced Hydrogen atoms are babanced-
4) $03? eHx0 —> 0g +2444 90O— OD (UMnod 48H M450
charges are balanced charges are balanced > 1”
Ox5 5 503% + 5 H20 —> S504*+ tH + Loe”
@Oxe 3 eMnog +t6H*eloe > 2MN*? 4+ PHeO-
SG Soy OHeD HIM
SS ee oe 2 ea
4 © = 2rMnog +5503 + 6Ht~y 2Mnt
—
25)'} A carbon compound contains 128% oF C, W414 OF H,
|} (6:54 of Br the M-wt of the Compound js 184% °4
calculate the molecular Formula
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5796)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1091)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (589)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- JR Botany Ipe Baby BulletDocument71 pagesJR Botany Ipe Baby Bulletangadibalajithkumar91% (11)
- Chemistry Material How To Get 60Document60 pagesChemistry Material How To Get 60angadibalajithkumarNo ratings yet
- JR English Bullet PDFDocument72 pagesJR English Bullet PDFasaabhi9059100% (3)
- English Ipe First Year Key Points of The Public ExaminationsDocument11 pagesEnglish Ipe First Year Key Points of The Public ExaminationsangadibalajithkumarNo ratings yet
- JR Inter Botany IPE Model Paper 3Document2 pagesJR Inter Botany IPE Model Paper 3angadibalajithkumar100% (1)
- JR - Inter IPE Zoology Model Paper 3Document2 pagesJR - Inter IPE Zoology Model Paper 3angadibalajithkumarNo ratings yet
- JR Physics Imp Vsaq's 2023-24Document3 pagesJR Physics Imp Vsaq's 2023-24angadibalajithkumarNo ratings yet
- JR - Inter Zoology Model Paper 4Document2 pagesJR - Inter Zoology Model Paper 4angadibalajithkumarNo ratings yet
- JR - Inter Ipe Chemistry Model Paper 2Document2 pagesJR - Inter Ipe Chemistry Model Paper 2angadibalajithkumarNo ratings yet
- JR - Inter Zoology Model Paper 2Document2 pagesJR - Inter Zoology Model Paper 2angadibalajithkumarNo ratings yet
- JR - Inter Ipe Zoology Model Paper 1Document1 pageJR - Inter Ipe Zoology Model Paper 1angadibalajithkumarNo ratings yet
- JR - Inter Botany Model Paper 2Document1 pageJR - Inter Botany Model Paper 2angadibalajithkumarNo ratings yet
- JR - Inter Botany Ipe Model Paper 1Document2 pagesJR - Inter Botany Ipe Model Paper 1angadibalajithkumarNo ratings yet
- JR - Inter Physics Model Paper 2Document2 pagesJR - Inter Physics Model Paper 2angadibalajithkumarNo ratings yet
- JR - Inter Ipe Chemistry Model Paper 1Document2 pagesJR - Inter Ipe Chemistry Model Paper 1angadibalajithkumarNo ratings yet
- JR - Inter Ipe Physics Model Paper 1Document1 pageJR - Inter Ipe Physics Model Paper 1angadibalajithkumarNo ratings yet
- Baby Bullet-Q JR Physics New EditionDocument68 pagesBaby Bullet-Q JR Physics New Editionangadibalajithkumar90% (10)
- JR Physics Ipe Imp QuestionsDocument53 pagesJR Physics Ipe Imp QuestionsangadibalajithkumarNo ratings yet
- Zoology Baby Bullet Inter JuniorDocument78 pagesZoology Baby Bullet Inter Juniorangadibalajithkumar92% (13)
- English Grammer Tenses NotesDocument13 pagesEnglish Grammer Tenses NotesangadibalajithkumarNo ratings yet