Professional Documents
Culture Documents
Carnot Cycle
Uploaded by
Rana krupalOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Carnot Cycle
Uploaded by
Rana krupalCopyright:
Available Formats
3.
5 Carnot cycle: p-V diagram and T-S diagram 45
3.5 Carnot cycle: p-V diagram and T-S diagram
In what follows we discuss one of the most important concepts of thermodynamics, the Carnot cycle. In general in
a circular process the thermodynamic system passes several exchange points until it ends up in its starting point.
It is important to note that for different branches the surrounding can change, i.e. energy in terms of heat or work
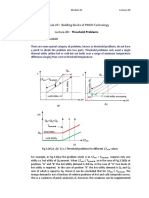
can be transferred from different heat reservoirs. Fig. 3.2 a) illustrates a Carnot cycle. This is a combination of
reversible isothermal and adiabatic expansion and compression of a perfect gas:
A → B: q1 is isothermally (@T1 ) transferred from the first heat reservoir to the system (w1 = area ABba)
B → C: adiabatic expansion reducing the temperature from T1 to T2 (w2 = area BCcb)
C → D: q2 is isothermally (@T2 ) transferred from the system to the second heat reservoir (w3 = area DCcd)
D → A: adiabatic compression, increasing temperature from T2 to T1 (w4 = area ADda)
The gas becomes cold and hot after adiabatic expansion and compression, respectively. The area in the p − V
diagram represents expansion work w. Now we discuss the T − S diagram of a Carnot cycle as shown in Fig. 3.2
b). Since T dS = δqrev the area in the T − S diagram represents qrev . So no contribution to qrev comes from the
adiabatic processes. For the isothermal processes we have w = −q, so the work done (output) is given by the area
ABCD.
A → B: heat input (−q1 = w1 = area ABS2 S1 )
B → C: q = 0
C → D: heat output (−q3 = w3 = area CS2 S1 D)
D → A: q = 0
Obviously larger T -differences and large S-differences are needed for efficient processes (i.e. more work can be
performed). The w-contributions from the adiabatic steps are canceling out. Thus only the isothermal steps
contribute to heat and by that to work.
Adiabate
q1, heat input
A: T1 V1
pressure, p
A B
T1
Work
Hot output
Adiabate D C
T2
D: T2 V4 B: T1 V2
Cold
Isotherms
C: T2 V3
a d b c S1 S2
a) Volume,V b) q2, heat output
Figure 3.2: Carnot cycle, i.e. a combination of isothermal and adiabatic compression and expansion. a) p − V
diagram; b) T − S diagram.
You might also like
- Cycling Science by Stephen S. Cheung & Mikel ZabalaDocument569 pagesCycling Science by Stephen S. Cheung & Mikel ZabalaYeboto Piratadelcaribe100% (6)
- FastenersDocument178 pagesFastenersthulasi_krishna100% (6)
- Lecture 7 (Types of Welding)Document22 pagesLecture 7 (Types of Welding)Syed Ahmed RazaNo ratings yet
- Test Your C SkillsDocument8 pagesTest Your C SkillsBharadwaj SubramaniamNo ratings yet
- Measure Uncertainity in Torque WrenchDocument11 pagesMeasure Uncertainity in Torque WrenchAnonymous uXdS9Y7100% (2)
- Solution Manual for an Introduction to Equilibrium ThermodynamicsFrom EverandSolution Manual for an Introduction to Equilibrium ThermodynamicsNo ratings yet
- How Squat, Bank and Bank Cushion Effects Influence Ships in Restricted Waters?Document7 pagesHow Squat, Bank and Bank Cushion Effects Influence Ships in Restricted Waters?shubham100% (1)
- Butyraldehyde Final ReportDocument85 pagesButyraldehyde Final ReportRana krupal100% (1)
- Physics PDFDocument517 pagesPhysics PDFParth ShahNo ratings yet
- 3 Oil and Gas Separation Design Manual by C Richard SivallsDocument63 pages3 Oil and Gas Separation Design Manual by C Richard SivallsSemih Özsağıroğlu100% (1)
- 25 Cross Flow Heat ExchangerDocument13 pages25 Cross Flow Heat Exchangerananth2012No ratings yet
- PT326Document12 pagesPT326Asok Suthakar50% (2)
- Current Source InverterDocument16 pagesCurrent Source Inverterjp-sharma100% (1)
- Steam and Gas Tables with Computer EquationsFrom EverandSteam and Gas Tables with Computer EquationsRating: 3 out of 5 stars3/5 (2)
- Kerson Huang - Introduction To Statistical PhysicsDocument440 pagesKerson Huang - Introduction To Statistical PhysicsMaher Al oboodyNo ratings yet
- Clausius-Clapeyron Equation: Liquid SolidDocument19 pagesClausius-Clapeyron Equation: Liquid Solidzaheer abbasNo ratings yet
- Carnot's TheorreDocument12 pagesCarnot's Theorremr shantosNo ratings yet
- Heat Transfer Lab: Me8512-Thermal Engineering LabDocument55 pagesHeat Transfer Lab: Me8512-Thermal Engineering LabVinoNo ratings yet
- 3 - Flow Processes - Carnot - CycleDocument37 pages3 - Flow Processes - Carnot - Cyclemikeclarke1500No ratings yet
- Heat Exchanger DesignDocument7 pagesHeat Exchanger DesignjanakNo ratings yet
- Design and Fabrication of Heat Exchanger For Co-Generation: February 2018Document7 pagesDesign and Fabrication of Heat Exchanger For Co-Generation: February 2018Francisco Rojo TrujilloNo ratings yet
- MODULE 3-ICE CyclesDocument4 pagesMODULE 3-ICE CyclesNigel Ceasar SilvaNo ratings yet
- Key Words:: Module 03: Building Blocks of PINCH Technology Lecture 06: Cold Composite CurvesDocument4 pagesKey Words:: Module 03: Building Blocks of PINCH Technology Lecture 06: Cold Composite Curvesbhavna23No ratings yet
- Iit Jam Thermodynamics PDFDocument16 pagesIit Jam Thermodynamics PDFAthira R GNo ratings yet
- Hot and Cold Pinch PDFDocument7 pagesHot and Cold Pinch PDFYatharth SahuNo ratings yet
- Thermal Conductivity (Ex-1)Document2 pagesThermal Conductivity (Ex-1)Yash RanaNo ratings yet
- 1.thermodynamics 21 30Document10 pages1.thermodynamics 21 30Aqsa KanwalNo ratings yet
- Set 5 Cat-C SolutionDocument4 pagesSet 5 Cat-C SolutionMohammad AfifNo ratings yet
- Building Blocks of PINCH Technology-Lect 8 PDFDocument8 pagesBuilding Blocks of PINCH Technology-Lect 8 PDFSukanta K. DashNo ratings yet
- INSP CHAMPS 2022 THERMODYNAMICS FinalDocument20 pagesINSP CHAMPS 2022 THERMODYNAMICS FinalSubham KumarNo ratings yet
- Thermodynamic CyclesDocument1 pageThermodynamic CyclesSalvador Monroy GalvánNo ratings yet
- Gaseous State and Thermodynamics - Level 3 - SolutionsDocument10 pagesGaseous State and Thermodynamics - Level 3 - Solutionsdemolition squadNo ratings yet
- Visit Us At: WWW - Nodia.co - In: GATE Previous Year Solved Paper For Mechanical EngineeringDocument1 pageVisit Us At: WWW - Nodia.co - In: GATE Previous Year Solved Paper For Mechanical EngineeringSameerChauhanNo ratings yet
- Guided Revision On Heat Transfer (Eng)Document6 pagesGuided Revision On Heat Transfer (Eng)jthyfgdNo ratings yet
- 1.thermodynamics 31 40Document10 pages1.thermodynamics 31 40Aqsa KanwalNo ratings yet
- KTG & Thermodynamics (QB) For-FDocument8 pagesKTG & Thermodynamics (QB) For-FRaju SinghNo ratings yet
- SA - ThermodynamicsDocument9 pagesSA - Thermodynamicsdheeraj831No ratings yet
- NK C SI R: Thermal Physics, Home Work Sheet-2Document1 pageNK C SI R: Thermal Physics, Home Work Sheet-2bhadrabijumohan2007No ratings yet
- IZhO 2023 Theory Eng SolDocument19 pagesIZhO 2023 Theory Eng SolVic YassenovNo ratings yet
- PINCH ANALYSIS Part 3 - Units and Area Targeting - SupertargetingDocument21 pagesPINCH ANALYSIS Part 3 - Units and Area Targeting - SupertargetingFrank A.No ratings yet
- Chapter 15 - Pinch Technology Heat Exchange Networks: Chemical Process Design West Virginia UniversityDocument42 pagesChapter 15 - Pinch Technology Heat Exchange Networks: Chemical Process Design West Virginia Universitysumit dekateNo ratings yet
- Condution Holman 10th-EdDocument167 pagesCondution Holman 10th-EdLarysa SaganNo ratings yet
- Condution Holman 10th-Ed PDFDocument167 pagesCondution Holman 10th-Ed PDFLizbeth Abril100% (1)
- Chapter 4.2 4.3 Part 2Document25 pagesChapter 4.2 4.3 Part 2splint6969No ratings yet
- Facts and Formulae SheetDocument7 pagesFacts and Formulae SheethenryNo ratings yet
- CL 5002-Chemical Engineering Laboratory Ii Conduction: (A) Heat Transfer Through Composite Wall (B) Heat Transfer Though Lagged PipeDocument7 pagesCL 5002-Chemical Engineering Laboratory Ii Conduction: (A) Heat Transfer Through Composite Wall (B) Heat Transfer Though Lagged PipeAbhishek Raj ShekharNo ratings yet
- استنتاج2Document30 pagesاستنتاج2mohamed orifNo ratings yet
- Lecture 2aDocument15 pagesLecture 2aKazem OsailyNo ratings yet
- Apparatus DesignDocument22 pagesApparatus Designmlkn tfraNo ratings yet
- Dual-Duct Constant-Volume System Analysis PDFDocument3 pagesDual-Duct Constant-Volume System Analysis PDFZaid Tariq AlabiryNo ratings yet
- Cooler (E-216)Document26 pagesCooler (E-216)Suci Nur RachmiNo ratings yet
- HandtDocument3 pagesHandtvanuNo ratings yet
- Heat Engine: - Sources of Heat Include The Combustion of Coal, Petroleum or Carbohydrates and Nuclear ReactionsDocument27 pagesHeat Engine: - Sources of Heat Include The Combustion of Coal, Petroleum or Carbohydrates and Nuclear ReactionsYeshua YeshaNo ratings yet
- 6.1. Introduction To The Second Law of Thermodynamics and Carnot EngineDocument18 pages6.1. Introduction To The Second Law of Thermodynamics and Carnot Enginelitebele litebeleNo ratings yet
- L4 - L7 Chapter-1Document8 pagesL4 - L7 Chapter-1niggsNo ratings yet
- 1 Integer 2008 To 11 Aits Pt+OtDocument4 pages1 Integer 2008 To 11 Aits Pt+OtTEJASSINGH ARORANo ratings yet
- Student Copy - Caps - 20Document7 pagesStudent Copy - Caps - 20Sujal KumarNo ratings yet
- 15-Heat Exchangers 1Document21 pages15-Heat Exchangers 1joshuaterence666No ratings yet
- Meherwan P Boyce - Gas Turbine Engineering Handbook-Elsevier Butterworth-Heinemann (2012) 27Document5 pagesMeherwan P Boyce - Gas Turbine Engineering Handbook-Elsevier Butterworth-Heinemann (2012) 27amir moniriNo ratings yet
- HT Lab Manual - Cycle 2Document22 pagesHT Lab Manual - Cycle 2konda Sasi Snehith reddyNo ratings yet
- Designing of HEN: - Designing A Heat Exchanger NetworkDocument12 pagesDesigning of HEN: - Designing A Heat Exchanger Networkshafeeque79100% (1)
- Conduction SteadyDocument33 pagesConduction Steadyguna sekaranNo ratings yet
- 7th ExamplesDocument6 pages7th ExamplesIuhence VergaraNo ratings yet
- Kelm 112Document6 pagesKelm 112Lakshay KunduNo ratings yet
- EPF4802 - Chapter 5 (Part 2) Utilities and Energy Efficient Design - NotesDocument21 pagesEPF4802 - Chapter 5 (Part 2) Utilities and Energy Efficient Design - NoteshidayantiemNo ratings yet
- 2 Thermal Conductivity of Metal RodDocument10 pages2 Thermal Conductivity of Metal RodMithilesh kumarNo ratings yet
- CondenserDocument1 pageCondenserhoangvubui4632No ratings yet
- Earth Overshoot Day 2023 Nowcast ReportDocument9 pagesEarth Overshoot Day 2023 Nowcast ReportRana krupalNo ratings yet
- Layout For Centralized Eme - Tankfarm - 20-02-24 - 4Document1 pageLayout For Centralized Eme - Tankfarm - 20-02-24 - 4Rana krupalNo ratings yet
- Jayant S Ullas - Praj IndustriesDocument1 pageJayant S Ullas - Praj IndustriesRana krupalNo ratings yet
- Layout For Centralized Eme - Tankfarm - 20-02-24 - 3Document1 pageLayout For Centralized Eme - Tankfarm - 20-02-24 - 3Rana krupalNo ratings yet
- Layout For Centralized Eme - Tankfarm - 20-02-24 - 5Document1 pageLayout For Centralized Eme - Tankfarm - 20-02-24 - 5Rana krupalNo ratings yet
- Safety Data Sheet: Tokyo Chemical Industry (India) Pvt. LTDDocument5 pagesSafety Data Sheet: Tokyo Chemical Industry (India) Pvt. LTDRana krupalNo ratings yet
- Potassium Hydroxide Pellets CAS No 1310-58-3: Material Safety Data Sheet Sds/MsdsDocument7 pagesPotassium Hydroxide Pellets CAS No 1310-58-3: Material Safety Data Sheet Sds/MsdsRana krupalNo ratings yet
- Softwares in Process EngineeringDocument7 pagesSoftwares in Process EngineeringRana krupalNo ratings yet
- Flame Arrestor Function and UsesDocument4 pagesFlame Arrestor Function and UsesRana krupalNo ratings yet
- Isye 6669 HW 2: 1 2 3 3 I 1 I 2 I 1 4 J 2 Ij I 3 T 1 X T! 1 K 1 2 K 1 3 I 1 I J 1 I+J 4 N 2 N+2 M N N MDocument2 pagesIsye 6669 HW 2: 1 2 3 3 I 1 I 2 I 1 4 J 2 Ij I 3 T 1 X T! 1 K 1 2 K 1 3 I 1 I J 1 I+J 4 N 2 N+2 M N N MMalik KhaledNo ratings yet
- NASA: 89235main TF-2004-16-DFRCDocument8 pagesNASA: 89235main TF-2004-16-DFRCNASAdocumentsNo ratings yet
- Escape Velocity PDFDocument5 pagesEscape Velocity PDFRatriNo ratings yet
- Physics Multiple Choice Questions and Answers PDF NotesDocument57 pagesPhysics Multiple Choice Questions and Answers PDF NotesQurrat ul AinNo ratings yet
- Medina (193253920) Problemset7 V1Document6 pagesMedina (193253920) Problemset7 V1shaunmedina0006No ratings yet
- Certification of Anti-Seismic Devices According To The European Standard EN 15129:2009: Tasks For Manufacturers and Notified BodiesDocument9 pagesCertification of Anti-Seismic Devices According To The European Standard EN 15129:2009: Tasks For Manufacturers and Notified BodiesRobby PermataNo ratings yet
- Instruction: Chapter Four: Imperfections in Solids Part OneDocument2 pagesInstruction: Chapter Four: Imperfections in Solids Part OnedebelaNo ratings yet
- 18-Soft Soil Creep Model - PlaxisDocument5 pages18-Soft Soil Creep Model - PlaxisVa Ni SkyNo ratings yet
- 54449913Document464 pages54449913nadbrahmaNo ratings yet
- ADMModule - STEM - GP12EDocument26 pagesADMModule - STEM - GP12EJhon Christian ManlangitNo ratings yet
- FS Phase 4 UT TE Planner - AY 2022-23Document1 pageFS Phase 4 UT TE Planner - AY 2022-23AyushNo ratings yet
- Staging and Control of Rockets and Missiles - PritamashutoshDocument9 pagesStaging and Control of Rockets and Missiles - PritamashutoshUma MageshwariNo ratings yet
- 2811 Jan 04MSDocument7 pages2811 Jan 04MSmichael hengNo ratings yet
- Heat Transfer in Composite WallDocument37 pagesHeat Transfer in Composite WallWong Hang SingNo ratings yet
- Practical No: 08 Title: Explanation of Mass Balance by Algebraic Equations Objective: To Acquaint Food Processing Operation by Mass Balance. TheoryDocument4 pagesPractical No: 08 Title: Explanation of Mass Balance by Algebraic Equations Objective: To Acquaint Food Processing Operation by Mass Balance. TheoryMuhammad Faisal AdreesNo ratings yet
- NotesDocument386 pagesNotesjovan avery dalluayNo ratings yet
- Magnetism PDFDocument4 pagesMagnetism PDFNiksslpadaNo ratings yet
- Class 9 Physics Worksheet FinalDocument5 pagesClass 9 Physics Worksheet FinalTwisha JainNo ratings yet
- Classical Mechanics LecturesDocument74 pagesClassical Mechanics LecturesMose DelPozzoNo ratings yet
- MEC111 Chapter3 PDFDocument36 pagesMEC111 Chapter3 PDFHaFiy HaZimNo ratings yet
- DfreDocument5 pagesDfreVishal MeenaNo ratings yet
- App 014 Combining KISSsys FEMDocument8 pagesApp 014 Combining KISSsys FEMalexNo ratings yet