Professional Documents
Culture Documents
Chem 9th MB Chapter No 04
Uploaded by
Safeer AhmedOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chem 9th MB Chapter No 04
Uploaded by
Safeer AhmedCopyright:
Available Formats
BUTT EDUCATIONAL SCIENCE ACADEMY
MB 9th
CLASS CH.NO
04
SUBJECT
Chemistry
MARKS
40
TIME
ALLOWED
65 minutes
NAME OF THE STUDENT ROLL NO:
DATE:-22January, 2024
Test Number: 15
SETION – B (SHORT QUESTIONS: MARKS)
Q.NO.02) ATTEMPT ANY FIVE QUSTIONS. (2 × 05 = 10)
i) Noble gases are least reactive. Comment.
ii) How do you distinguish between a double covalent and a triple covalent bond?
iii) Water has maximum density at 4oC. Give reason
iv) Define intermolecular forces; show these forces among HCI molecule.
v) Define covalent bond. Give example.
vi) Metals are good conductor of electricity. Why?
Q.NO.03) ATTEMPT ANY FIVE QUSTIONS. (2 × 05= 10)
i) What is coordinate covalent bond?
ii) Give the formation of the following molecule
i. NH3 ii. CH4
iii) What do you mean by malleability and ductility?
iv) Define octet rule and duplet rule? Give examples in each case.
v) State electron pool theory or Gas theory.
vi) Why ionic compounds have high melting and boiling point?
SETION – III (LONG QUESTIONS)
NOTE: ATTEMPT ANY ONE QUESTIONS ( 1 × 10 = 10 )
Q.NO.04 Explain major difference between non-polar and polar covalent bond.
b) Write any five properties covalent compounds.
Q.NP.05
Write a detailed note on Hydrogen Bonding?
b) Write a note on dipole-dipole forces. Give properties of dipole-dipole forces.
……………Compiled by Rana Safeer Ahmed……………
BUTT EDUCATIONAL SCIENCE ACADEMY
MB 9th
CLASS CH.NO
04
SUBJECT
Chemistry
MARKS
10
TIME
ALLOWED
10minutes
NAME OF THE STUDENT ROLL NO:
DATE:-22January, 2024
Test Number: 15
SECTION – A
Section – A is compulsory. All parts of this section are to be answered on this page and handed over to the Centre
Superintendent. Deleting/overwriting is not allowed. Do not use lead pencil.
Q.No.1 TICK ( ) THE CORRECT OPTION. i.e A/B/C/D
i. Valance of element of noble gases in general state:
A Three B Zero C One D Two
ii. The force of attraction between atom that hold them together in a molecule called:
A Ionic bond B Chemical bond C Metallic bond D Covalent bond
iii. The atom which provides the electron pair is formed:
A Accepter B Donor C Molecule D Atom
iv. When atom lose election the size of the atom became:
A Smaller B Larger C Medium D None
v. Which of the following molecules has a coordinate covalent bond:
A C2H6 B NaCl C HCI D AlCl3
vi. In H +δ O−δ hydrogen bond energy is ___ KJ/mol
A 25 B 45 C 13 D 15
vii. which of the following molecule has polar covalent bond?
A HCl B HF C H2 D NH3
viii. How many electrons are shared in a double covalent bond?
A Two B Three C Six D Two pair of
electrons
ix. which of the following molecules has a coordinate covalent bond?
A NaOH B NH4Cl C KCl D HBr
x. water has maximum density at?
A 0°C B 4°C C 100°C D 25°C
(THE END)
GOOD LUCK
You might also like
- A Textbook of Chemical Engineering Thermodynamics - K. V. Narayanan PDFDocument222 pagesA Textbook of Chemical Engineering Thermodynamics - K. V. Narayanan PDFRaj PatelNo ratings yet
- General ChemistryDocument4 pagesGeneral ChemistryKrizzia Anne ShengNo ratings yet
- Engineering Metallurgy MCQsDocument18 pagesEngineering Metallurgy MCQssaurabh bhange0% (2)
- Chem 9th MB Chapter No 01Document3 pagesChem 9th MB Chapter No 01Safeer AhmedNo ratings yet
- Chemistry 9th Model PaperDocument3 pagesChemistry 9th Model PaperHasnain Ahmad KhanNo ratings yet
- Chemistry 2nd Year Chapter 09Document14 pagesChemistry 2nd Year Chapter 09shahzad noorNo ratings yet
- 9th Class Annual Chemistry Paper Group B New PDFDocument2 pages9th Class Annual Chemistry Paper Group B New PDFAamir HabibNo ratings yet
- Chem 9th MB Chapter No 03Document3 pagesChem 9th MB Chapter No 03Safeer AhmedNo ratings yet
- TS JR (Pre-Final-2) (Chemstry Q P) Ex DT 17-04-2021Document2 pagesTS JR (Pre-Final-2) (Chemstry Q P) Ex DT 17-04-2021AbhiNo ratings yet
- CHP 2Document1 pageCHP 2Haseeb AhmadNo ratings yet
- Chemistry - JEE, J1 & JR. BIPC (E)Document1 pageChemistry - JEE, J1 & JR. BIPC (E)Mavuluri UmamaheshNo ratings yet
- Chem X Mid Term 21-22Document2 pagesChem X Mid Term 21-22salmanNo ratings yet
- Chem 9th MB Chapter No 05Document2 pagesChem 9th MB Chapter No 05Safeer AhmedNo ratings yet
- 9.chem Paper F.T 2017-18 AnsDocument4 pages9.chem Paper F.T 2017-18 AnsTanveer AhmedNo ratings yet
- Chemistry First HalfDocument4 pagesChemistry First Halfamnashabbir209No ratings yet
- Chemistry 1Document15 pagesChemistry 1Zamin Abbas KhanNo ratings yet
- Aspire Group of Colleges (Chakwal Campus)Document1 pageAspire Group of Colleges (Chakwal Campus)ASPIRE COLLEGE CHAKWAL CAMPUSNo ratings yet
- CHEM (1st) May19Document1 pageCHEM (1st) May19Hitakshi VermaNo ratings yet
- Delta Junior College: OH 4 Aq 2 S 2 S AqDocument3 pagesDelta Junior College: OH 4 Aq 2 S 2 S AqrammNo ratings yet
- 2nd Year CHEMISTRY CH Wise 2021 by 786 AcademyDocument14 pages2nd Year CHEMISTRY CH Wise 2021 by 786 AcademyAbdul Majeed Maitla100% (2)
- JR - Inter Ipe Chemistry Model Paper 2Document2 pagesJR - Inter Ipe Chemistry Model Paper 2angadibalajithkumarNo ratings yet
- 9 Chemistry Series Test # 2: Q. 1: Choose The Correct Option.Document3 pages9 Chemistry Series Test # 2: Q. 1: Choose The Correct Option.CosmeriesNo ratings yet
- ChemistryDocument1 pageChemistryIrfanullahNo ratings yet
- 9th 4th CHPDocument2 pages9th 4th CHPMuhammad Qadir RafiqueNo ratings yet
- Comprehensive-Chemistry PaperDocument4 pagesComprehensive-Chemistry PaperUmar ZulfiqarNo ratings yet
- BSC I Year - Chemistry Paper-I - 2015Document3 pagesBSC I Year - Chemistry Paper-I - 2015Urvi KaleNo ratings yet
- Cambridge International Examinations Cambridge International General Certificate of Secondary EducationDocument16 pagesCambridge International Examinations Cambridge International General Certificate of Secondary EducationDenver DemisNo ratings yet
- Int 1 Chemistry Set 2Document2 pagesInt 1 Chemistry Set 2Krk PrasadNo ratings yet
- 2nd Quarterly Exam in Science 9Document4 pages2nd Quarterly Exam in Science 9Vee Jay BlanciaNo ratings yet
- 7 3 Q4. I) Give Characteristics of Homologous Series. 4 3 Q5. I) Explain The Zeolite Process For Removal of Hardness of Water. 6 4Document2 pages7 3 Q4. I) Give Characteristics of Homologous Series. 4 3 Q5. I) Explain The Zeolite Process For Removal of Hardness of Water. 6 4Global College of Engineering TechnologyNo ratings yet
- 9th Model Paper Karachi ChemistryDocument2 pages9th Model Paper Karachi ChemistryAkber AnwarNo ratings yet
- Chem 9thDocument1 pageChem 9thnabeelaNo ratings yet
- Full Prelim Chem - 20-01-2022Document15 pagesFull Prelim Chem - 20-01-2022EZ SHaikhNo ratings yet
- JR Ipe - Chemistry PDF - Set-3Document1 pageJR Ipe - Chemistry PDF - Set-3udaysrinivasNo ratings yet
- Chemistry Model Paper 1Document2 pagesChemistry Model Paper 1bsahil2007No ratings yet
- Paper Chemistry Class 9 A: Time: 15min Objective Type Total Marks: 12Document2 pagesPaper Chemistry Class 9 A: Time: 15min Objective Type Total Marks: 12CosmeriesNo ratings yet
- 9th ChemistryDocument2 pages9th ChemistryMohsin AliNo ratings yet
- Chemistry 1st Year EMDocument2 pagesChemistry 1st Year EMJanagam LavanyaNo ratings yet
- Chemistry Pre Final 1 & 2 PapersDocument12 pagesChemistry Pre Final 1 & 2 PaperskayNo ratings yet
- 11BS104 Engineering ChemistryDocument1 page11BS104 Engineering ChemistryVenkat SaiNo ratings yet
- Cameroon General Certificate of Education Board 0515 Chemistry 1Document6 pagesCameroon General Certificate of Education Board 0515 Chemistry 1Talatouremi FruNo ratings yet
- Chemistry XIIDocument134 pagesChemistry XIIVenkitaraj K PNo ratings yet
- Federal Govt. Educational Institution KarachiDocument1 pageFederal Govt. Educational Institution KarachiSyed Muhammad Shamail EjazNo ratings yet
- Chemistry 1st Year Set-1 (English Medium) 2021 Guess PaperDocument2 pagesChemistry 1st Year Set-1 (English Medium) 2021 Guess PaperPawan Kalyan JpkNo ratings yet
- Mock Test IX Chemistry (Sep 2023)Document1 pageMock Test IX Chemistry (Sep 2023)abdullah OlakhNo ratings yet
- AP Inter I Year Chemistry (EM) 2020 Model Paper-1Document2 pagesAP Inter I Year Chemistry (EM) 2020 Model Paper-1Mohammed AliNo ratings yet
- Chemistry - J2, J3 & JR. BIPC (G)Document1 pageChemistry - J2, J3 & JR. BIPC (G)Mavuluri UmamaheshNo ratings yet
- Engg - Chemistry (CHM-101) PDFDocument2 pagesEngg - Chemistry (CHM-101) PDFRahul PinnamaneniNo ratings yet
- (TS) Junior Chemistry Pre Final Examination (Batch - I) (19!04!2022)Document2 pages(TS) Junior Chemistry Pre Final Examination (Batch - I) (19!04!2022)Artist GamingNo ratings yet
- Chemistry 9th 1st Half Book 2020-21Document2 pagesChemistry 9th 1st Half Book 2020-21noumanwaqarNo ratings yet
- CHEMISTRY Model Paper-3Document3 pagesCHEMISTRY Model Paper-3bsahil2007No ratings yet
- CHEMISTRY Model Paper-2Document3 pagesCHEMISTRY Model Paper-2bsahil2007No ratings yet
- Federal Board SSC-1 Chemistry Test # 1: Section-B (Marks 18)Document2 pagesFederal Board SSC-1 Chemistry Test # 1: Section-B (Marks 18)Sohail HameedNo ratings yet
- Section A: Chemical BondingDocument2 pagesSection A: Chemical BondingD91Soham ChavanNo ratings yet
- Btech 1 Sem Chemistry Kas 102 2018 19Document2 pagesBtech 1 Sem Chemistry Kas 102 2018 19Viraj RuhelaNo ratings yet
- Sr. Inter Chemistry IVDocument2 pagesSr. Inter Chemistry IVYuga Tejeshwar ReddyNo ratings yet
- Atoms & MoleculesDocument2 pagesAtoms & MoleculesNavas KappilNo ratings yet
- Uppp2 Sem 1 2017Document9 pagesUppp2 Sem 1 2017WWZNo ratings yet
- Class 1st Chemistry Test Unit FullDocument5 pagesClass 1st Chemistry Test Unit FullAadNo ratings yet
- Paper Chemistry Class 9 A: Time: 15min Objective Type Total Marks: 12Document2 pagesPaper Chemistry Class 9 A: Time: 15min Objective Type Total Marks: 12CosmeriesNo ratings yet
- Chemistry PaperDocument2 pagesChemistry PaperHassan RazaNo ratings yet
- CFD Model and Control of A SMR ReactorDocument15 pagesCFD Model and Control of A SMR Reactorcholila_tamzysiNo ratings yet
- 2008 CSEC Physics Specimen P1Document14 pages2008 CSEC Physics Specimen P1AyanaNurseNo ratings yet
- Earth Science: Quarter 2 - Module 4: MetamorphismDocument8 pagesEarth Science: Quarter 2 - Module 4: MetamorphismBea CabacunganNo ratings yet
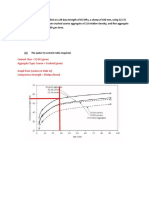
- Cement Class 52.5R (Given) Aggregate Type: Coarse Crushed (Given) Graph From (Lecture 8, Slide 22) Compressive Strength 55mpa (Found)Document11 pagesCement Class 52.5R (Given) Aggregate Type: Coarse Crushed (Given) Graph From (Lecture 8, Slide 22) Compressive Strength 55mpa (Found)Shael BridgelalNo ratings yet
- Summative Test 2 Science 7 (4 Quarter)Document2 pagesSummative Test 2 Science 7 (4 Quarter)Alvin GultiaNo ratings yet
- Crisis Summer Report 200311Document34 pagesCrisis Summer Report 200311zelmiinaNo ratings yet
- Combustion Properties of BiomassDocument30 pagesCombustion Properties of BiomassvolteanuNo ratings yet
- Boron Does Increase The Hardenability of The Steel Whenever It Is Present in The Steel Mix UpDocument4 pagesBoron Does Increase The Hardenability of The Steel Whenever It Is Present in The Steel Mix UpRanbir singhNo ratings yet
- Final Exam 20172018 Sem 2Document10 pagesFinal Exam 20172018 Sem 2Abdulrahman DesoukyNo ratings yet
- Steam Generation Through Solar Radiation by Optimizing Collector Design"Document62 pagesSteam Generation Through Solar Radiation by Optimizing Collector Design"shahjamalNo ratings yet
- GU - Installazione Quadri MT (EN) - 1VCP000630 - 1605Document18 pagesGU - Installazione Quadri MT (EN) - 1VCP000630 - 1605AulinoNo ratings yet
- HW2 SolutionDocument4 pagesHW2 SolutiontsengullerNo ratings yet
- Department of Mechanical Engineering Indian Institute of Technology Kanpur KANPUR-208016 (INDIA) CAD of Thermal Systems (ME 648) Home Assignment - 12Document1 pageDepartment of Mechanical Engineering Indian Institute of Technology Kanpur KANPUR-208016 (INDIA) CAD of Thermal Systems (ME 648) Home Assignment - 12AsheeshKumarNo ratings yet
- 300817Document4 pages300817Mahesh SinghNo ratings yet
- Chapter 19 Solved ProblemsDocument33 pagesChapter 19 Solved Problemsİzem OsmaNo ratings yet
- Clay Minerals and Soil StructureDocument81 pagesClay Minerals and Soil StructureAthiyo MartinNo ratings yet
- Syllabus in 3Document5 pagesSyllabus in 3jb umusigNo ratings yet
- Book of AbstractsDocument196 pagesBook of AbstractsRaletic GoranNo ratings yet
- Experiment NO. 1-Physical and Chemical ChangeDocument2 pagesExperiment NO. 1-Physical and Chemical ChangeJenniferNo ratings yet
- Chemistry DPPDocument16 pagesChemistry DPPAyan KhanNo ratings yet
- Public Private Partnership of Hydro Projects in NepalDocument24 pagesPublic Private Partnership of Hydro Projects in NepalYashaswi GhimireNo ratings yet
- Reflection 456Document1 pageReflection 456elarzzzzNo ratings yet
- Eals Lesson 2 Student'sDocument15 pagesEals Lesson 2 Student'sMai SasaNo ratings yet
- Solar Vapour Absorption SystemDocument7 pagesSolar Vapour Absorption SystemMir Aqueel AliNo ratings yet
- Reservoir Fluids Properties V2Document345 pagesReservoir Fluids Properties V2ossama farghlyNo ratings yet
- 8 1 HEAT TRANSFER 2016 QuestionaireDocument3 pages8 1 HEAT TRANSFER 2016 QuestionaireLourdes CagungunNo ratings yet
- FINALSDocument32 pagesFINALSNathasha Marie BanalNo ratings yet
- NANO-Production-Manifestation-RESOLUTIONS AND PROTECTIONS PDFDocument358 pagesNANO-Production-Manifestation-RESOLUTIONS AND PROTECTIONS PDFBryan396100% (1)