Professional Documents
Culture Documents
Coordination WS Key
Uploaded by
Deena chemistOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Coordination WS Key
Uploaded by
Deena chemistCopyright:
Available Formats
PINNAACLE CLASSES
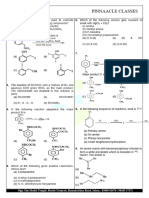
1. (b)
CuSO 4 +4 NH 3 →[Cu( NH 3 )4 ]SO 4
10. (c) It gives precipitate with
AgNO
3 it means it given Cl
−
2. (b) Oxalate is a bidentate ligand hence forms a chelate. ion in the solutions. Since conductivity corresponds to two
−
ions, it shows one Cl is outside the coordination sphere. The
structure will be
[Cr ( H 2 O )4 Cl 2 ]Cl→[Cr ( H 2 O)4 Cl 2 ]+ +Cl−
−
3. (a) Oxidations state of Mn in
[ MnO 4 ] = +7, hence it does AgNO 3 +Cl− → AgCl +NO −3
not have any d-electron present in it. White ppt
0 0
Mn7 + (Z = 25) : [ Ar ]4 s 3d
[Co( NH 3 )5 SO 4 ]Br →[Co( NH 3 )5 SO4 ]+ +Br −
(d) In the complex linkage, geometrical and optical isomerism 11.
X
4.
are possible. AgNO 3 +Br− → AgBr +NO−3
pale yellow
[Co(C 2 O4 )3 ] 3− [Co( NH 3 )5 Br ]SO 4 →[Co( NH 3 )5 Br ]2+ +SO 2−
4
5. (d) −
Oxidations state of Co = +3 BaCl 2 + SO2−
4 →BaSO 4 +2 Cl
White ppt
Co 3+ (Z=27 ):3 d 6
Since
C 2 O2−
4 is a strong filed ligand pairing will take place. 2 . 675
CoCl 3 . 6 NH 3 = =0 . 01
12. (b) No. of moles of 267 .5
4 . 78
= =0. 03
No. of moles of AgCl 143 . 5
6. (a) Electronic configurations of Co
3+
=3 d 6 0 . 01 moles of the complex CoCl 3 . 6 NH 3 gives 0 . 03
Since
[Co(CN )6 ]3− AgNO .
3 it implies the 3
moles of AgCl on treatment with
chloirde ion are ionisable, in the complex. Thus, that formula
of the complex is
[Co( NH 3 )6 ]Cl 3 .
No. of unpaired electrons = 0.
Hence, shows no paramagnetism. 13. (a) SCN and
− NO−
2 are ambidentate ligand since they have
more than one donor atoms to attach to the central metal
6 atom.
7. (c) In d (low spin) electrons get paired up to make two d-
orbitals empty. Hydridisations is d 2 sp 3 (octahedral) and the 14. (a) Potassium pentacyanonitrosylferrate (II)
complex is low spin.
15. (b) The ligand are interchanged in both the cationic and
8. (c) Cr is in zero oxidations state. anionic entities of different metal ions present in a complex to
form coordination isomers.
16. (c) Primary valencies are also known as oxidation state.
K 2 [ Ni (CN )4 ] , 2+ x−4=0⇒ x =+ 2
No. of unpaired electrons
=0 17. (a)
μ= √0 (0+2)=0 When metal is in low oxidation state then it
forms complexes when ligands have good -
accepting character
9. (a) Higher the number of ions in the solutions, higher is the
conductivity.
18. (b) Complexes in which a metal is bound to more than one
No. of ion :
[Co( NH 3 )3 Cl 3 ]=0 ;
[Co( NH 3 ) 4 Cl 2 ]Cl=2 kind of donor groups, e.g., are known
as heteroleptic. Complexes in which a metal is bound to only
[Co( NH 3 )5 Cl ]Cl 2 =3 ; [Co( NH 3 )6 ]Cl 3 =4
Opp. Om Shakti Temple, Beside Naturals, Ramakrishna Road, Salem : 83009 81676 / 98403 37371
PINNAACLE CLASSES
27. (c)
one kind of donor groups, e.g., are known [Co(NH3)6]+3 – d2sp3 diamagnetic
as homoleptic.
28. (a)
[FeF6]3-: Fe3+= 3d5 0<P
19. (d)
20. (c) Correct match is, P-(ii),Q-(i),R-(iii),S-(iv)
21. (d) : Triamminetriaqua Number of unpaired e- = 5 35 BM
chromium(III) chloride [CoF6]3-:Co3+ = 3d6 ( 0<P)
22. (a) (yellow) gave
when it is treated with excess silver nitrate solution.
23. (a)
Number of unpaired e- = 4 24 BM
[Co(C2O4)3]3- : Co3+ = 3d6 ( 0 > P)
24. (b) Both and are tetrahedral
Number of unpaired e- = 0
0 BM
geometry
25. (c) has geometrical isomers, cis and 29. (b)
trans. The increasing order of field strength of
ligands (according to spectrochemical
series)
S2- < C2O24 < NH3 < en < CO
30. (b)
[Co(NH3)5SO4]Br+AgNO3 AgBr
0.01 mol excess 0.01 Mol
[Co(NH3)5Br]SO4+BaCl2 BaSO4
26. (d) 0.01 mol excess 0.01 Mol
Crystal field theory introduce
spectrochemical series based upon the
experimental values of but can't explain
it's order. While other three points arc
explained by CFT. Specially when the CFSE
increases thermodynamic stability of the
complex increases.
Opp. Om Shakti Temple, Beside Naturals, Ramakrishna Road, Salem : 83009 81676 / 98403 37371
You might also like
- Coordination WSDocument3 pagesCoordination WSDeena chemistNo ratings yet
- C Sol Ch-18 Co-Ordination CompoundsDocument12 pagesC Sol Ch-18 Co-Ordination Compoundsmysoftinfo.incNo ratings yet
- Answers of Sample Paper 6 To 12 Chemistry 12Document15 pagesAnswers of Sample Paper 6 To 12 Chemistry 12Lucky tiwariNo ratings yet
- Surface Chemistry (C New)Document13 pagesSurface Chemistry (C New)Avi KedarrNo ratings yet
- JEE Main Coordination Compounds Important QuestionsDocument17 pagesJEE Main Coordination Compounds Important Questionsixgreenprakharanjana16No ratings yet
- MCQs 1Document6 pagesMCQs 1VVA. .S0603No ratings yet
- Solutions To Mock IIT Advanced Test - 1/JEE-2018/Paper - 2: (Chemistry)Document10 pagesSolutions To Mock IIT Advanced Test - 1/JEE-2018/Paper - 2: (Chemistry)rajeshNo ratings yet
- Chemistry QP5Document5 pagesChemistry QP5Jinendra UvarajNo ratings yet
- Coordination Compounds - DTS 1 Adv (Archive) SolDocument4 pagesCoordination Compounds - DTS 1 Adv (Archive) SolRudra guptaNo ratings yet
- Chemistry: Coordination Compound Answer KeyDocument13 pagesChemistry: Coordination Compound Answer KeyDhruv KuchhalNo ratings yet
- Document PDF 467Document11 pagesDocument PDF 467exponential spiralNo ratings yet
- Chemistry Solutions: 1-YEAR Final Test Paper-2Document6 pagesChemistry Solutions: 1-YEAR Final Test Paper-2Vikas SinghNo ratings yet
- Coordination Chemistry TestDocument3 pagesCoordination Chemistry TestSabitra Rudra100% (1)
- Problem Xii emDocument34 pagesProblem Xii emAjayNo ratings yet
- Post-Lab 4 Qualitative Analysis-SolutionsDocument7 pagesPost-Lab 4 Qualitative Analysis-SolutionsUzo Paul NwabuisiNo ratings yet
- OKS Seminar04Document17 pagesOKS Seminar04KrenarNo ratings yet
- Chem Academy: Exercise - IDocument14 pagesChem Academy: Exercise - IHamit RanaNo ratings yet
- Chemistry - Cet 2021 - Version Code - B2 Solutions: Ans (C)Document15 pagesChemistry - Cet 2021 - Version Code - B2 Solutions: Ans (C)Swati NaikNo ratings yet
- The Transition Elements: Practice ExamplesDocument15 pagesThe Transition Elements: Practice Exampleskennethleo69No ratings yet
- 03-Coordination Chemistry - (Solution) - FinalDocument8 pages03-Coordination Chemistry - (Solution) - FinalAbhishek RavirajNo ratings yet
- Ionic EquilibriumDocument2 pagesIonic Equilibriumpinnaacleclasses salemNo ratings yet
- SP Print OutDocument39 pagesSP Print Outbruno we dont talk aboutNo ratings yet
- Jee Main 06 April 2023 Shift 1 Chemistry Memory Based Paper Solution - PHPDocument9 pagesJee Main 06 April 2023 Shift 1 Chemistry Memory Based Paper Solution - PHPAshish JhaNo ratings yet
- Latih Tubi Kimia Kumbe Matriculation Chemistry 2023Document7 pagesLatih Tubi Kimia Kumbe Matriculation Chemistry 2023Intan NoraisyahNo ratings yet
- Coordination CompoundsDocument12 pagesCoordination Compoundspinnaacleclasses salemNo ratings yet
- Chapter 9 Coordination CompoundsDocument10 pagesChapter 9 Coordination CompoundsDipti GuptaNo ratings yet
- Co-Ordination Compound Ex-4 Solution For Vedantu TatvaDocument9 pagesCo-Ordination Compound Ex-4 Solution For Vedantu TatvaAbhinav ThapliyalNo ratings yet
- Level - I: Solutions (Set-1)Document14 pagesLevel - I: Solutions (Set-1)Dwi RomadhonNo ratings yet
- N2016 H2 P1 Solns For UploadingDocument8 pagesN2016 H2 P1 Solns For UploadingjkNo ratings yet
- Solution 1277533Document8 pagesSolution 1277533subrat swainNo ratings yet
- Chapter 9 Ism 11e FinalDocument29 pagesChapter 9 Ism 11e FinalNathan VitorNo ratings yet
- CEMA-III-B (Organic & Inorgnaic)Document2 pagesCEMA-III-B (Organic & Inorgnaic)ARIJIT BHATTACHARYYANo ratings yet
- CH302 Assignments 2020BDocument5 pagesCH302 Assignments 2020BMike VhurinosharaNo ratings yet
- Solutions - Iit-Jee-2011: Code: 2: Chemistry Paper - 1Document5 pagesSolutions - Iit-Jee-2011: Code: 2: Chemistry Paper - 1Chinedu H. DuruNo ratings yet
- Sita RamDocument1 pageSita RamSampa MukherjeeNo ratings yet
- Results and Discussion 11Document4 pagesResults and Discussion 11fengyuhengNo ratings yet
- CBSE 12 Chemistry Solution Term2Document5 pagesCBSE 12 Chemistry Solution Term2R roseNo ratings yet
- Question Bank-Coordination CompoundsDocument3 pagesQuestion Bank-Coordination CompoundsMohamed zidan khanNo ratings yet
- Solutions To Mock IIT Advanced Test - 1/JEE-2018/Paper - 1: (Chemistry)Document12 pagesSolutions To Mock IIT Advanced Test - 1/JEE-2018/Paper - 1: (Chemistry)rajeshNo ratings yet
- SUPER REVISION - Coordination CompoundsDocument2 pagesSUPER REVISION - Coordination CompoundsChristopher NolanNo ratings yet
- Exercise With Ans FinalDocument24 pagesExercise With Ans Finald anjilappa25% (4)
- Bindura University of Science Education Chemistry DepartmentDocument3 pagesBindura University of Science Education Chemistry DepartmentdestinyyNo ratings yet
- CH 5 Coordination CompoundsDocument22 pagesCH 5 Coordination CompoundsNafeesNo ratings yet
- BITSAT Practice Paper - March 2016 XI XII 1 PDFDocument7 pagesBITSAT Practice Paper - March 2016 XI XII 1 PDFCHANDRA DEYNo ratings yet
- CLS JEEAD-19-20 XII Che Target-3 Level-1 Chapter-9Document12 pagesCLS JEEAD-19-20 XII Che Target-3 Level-1 Chapter-9SUNANDAN GUPTANo ratings yet
- Unit 8 AP Chem - Organic and ComplexesDocument20 pagesUnit 8 AP Chem - Organic and ComplexesMinnie InarapmasNo ratings yet
- Coordination Chemistry PDFDocument4 pagesCoordination Chemistry PDFBudhaditya BanerjeeNo ratings yet
- Che Neet 5Document5 pagesChe Neet 5pinnaacleclasses salemNo ratings yet
- Chem Set 2Document2 pagesChem Set 2pranjalsapkota99No ratings yet
- Day-2 Chemical BondingDocument4 pagesDay-2 Chemical BondingpriyanshuNo ratings yet
- CH 5 Coordination CompoundsDocument53 pagesCH 5 Coordination Compoundsgerawop972No ratings yet
- CFT and Chelate Effect-IDocument65 pagesCFT and Chelate Effect-IHitesh vadherNo ratings yet
- JOiz 7 U5 JOIODocument5 pagesJOiz 7 U5 JOIOKrishna Mohan ShuklaNo ratings yet
- 12 Chemistry Impq CH09 Coordination Compounds 01Document7 pages12 Chemistry Impq CH09 Coordination Compounds 01Sudarshan PandeyNo ratings yet
- Coordination CompoundsDocument18 pagesCoordination CompoundsAksa Merlin ThomasNo ratings yet
- AL Chemistry 1996 Paper 1+2Document12 pagesAL Chemistry 1996 Paper 1+2api-3734333No ratings yet
- Coordination Compounds ExercisesDocument55 pagesCoordination Compounds Exercisesaamir siddiquiNo ratings yet
- 12.co Ordination CompoundsExerciseDocument34 pages12.co Ordination CompoundsExerciseMaster Of HakingNo ratings yet
- Main Group Metal Coordination Polymers: Structures and NanostructuresFrom EverandMain Group Metal Coordination Polymers: Structures and NanostructuresNo ratings yet
- DPT 31 Xii Centre Rasi Che Iit Key 07-12-23Document4 pagesDPT 31 Xii Centre Rasi Che Iit Key 07-12-23Deena chemistNo ratings yet
- Xii DPT Bot 29.03.24Document6 pagesXii DPT Bot 29.03.24Deena chemistNo ratings yet
- WPT Xi Centre Che Neet Key 10-12-23Document3 pagesWPT Xi Centre Che Neet Key 10-12-23Deena chemistNo ratings yet
- DPT 31 Xii Centre Rasi Che Iit 07-12-23Document4 pagesDPT 31 Xii Centre Rasi Che Iit 07-12-23Deena chemistNo ratings yet
- WPT Xi Rasi Che Neet Key 2-12-23Document2 pagesWPT Xi Rasi Che Neet Key 2-12-23Deena chemistNo ratings yet
- DPT 33 Centre Rasi Iit Jee Che Key 09-12-23Document4 pagesDPT 33 Centre Rasi Iit Jee Che Key 09-12-23Deena chemistNo ratings yet
- Electrochemistry 45 KeyDocument10 pagesElectrochemistry 45 KeyDeena chemistNo ratings yet
- DPT 31 Xii Centre Rasi Che Neet Key 07-12-23Document8 pagesDPT 31 Xii Centre Rasi Che Neet Key 07-12-23Deena chemistNo ratings yet
- Rasi WPT Xi Che Iit Key 01-1-1-24Document2 pagesRasi WPT Xi Che Iit Key 01-1-1-24Deena chemistNo ratings yet
- Revision Schedule 23-24Document22 pagesRevision Schedule 23-24Deena chemistNo ratings yet
- Xi Rasi Neet Che WPT QP 22.01.2024Document3 pagesXi Rasi Neet Che WPT QP 22.01.2024Deena chemistNo ratings yet
- LT RPT Jee Phy 18.02.24Document4 pagesLT RPT Jee Phy 18.02.24Deena chemistNo ratings yet
- LT DPT 15 Jee 21.02.2024 KeyDocument1 pageLT DPT 15 Jee 21.02.2024 KeyDeena chemistNo ratings yet
- LT DPT Jee Key 22.02.24Document1 pageLT DPT Jee Key 22.02.24Deena chemistNo ratings yet
- LT RPT2 Jee Che 18-02-24Document2 pagesLT RPT2 Jee Che 18-02-24Deena chemistNo ratings yet
- Xi ND Phy Iit CPT 19.02.24Document4 pagesXi ND Phy Iit CPT 19.02.24Deena chemistNo ratings yet
- Xi ND CPT ZoologyDocument4 pagesXi ND CPT ZoologyDeena chemistNo ratings yet
- WPT CRP Xi Che Neet Key 18-02-24Document6 pagesWPT CRP Xi Che Neet Key 18-02-24Deena chemistNo ratings yet
- WPT Xi Centre Che Neet Key 21-11-23Document4 pagesWPT Xi Centre Che Neet Key 21-11-23Deena chemistNo ratings yet
- Xi Rasi Phy Iit WPT 19.02.24 KeyDocument1 pageXi Rasi Phy Iit WPT 19.02.24 KeyDeena chemistNo ratings yet
- LT Jee DPT 15.02.24Document3 pagesLT Jee DPT 15.02.24Deena chemistNo ratings yet
- X ND WPT Che 1 17-10-22Document1 pageX ND WPT Che 1 17-10-22Deena chemistNo ratings yet
- Dptchem & Zoo01.2024Document2 pagesDptchem & Zoo01.2024Deena chemistNo ratings yet
- Jee GrandDocument16 pagesJee GrandDeena chemistNo ratings yet
- Xi CRP Neet Che WPT QP 31.12.2023Document3 pagesXi CRP Neet Che WPT QP 31.12.2023Deena chemistNo ratings yet
- CPT Rasi Xi Che NeetDocument5 pagesCPT Rasi Xi Che NeetDeena chemistNo ratings yet
- Physiology - Regulation of Body TemperatureDocument35 pagesPhysiology - Regulation of Body TemperatureGhaidaa Sadeq100% (2)
- Levall 28: Product Data SheetDocument2 pagesLevall 28: Product Data SheetEfereonNo ratings yet
- How To Stay Fit and Healthy at Any Age 18 99 and Have Fun Doing ItDocument81 pagesHow To Stay Fit and Healthy at Any Age 18 99 and Have Fun Doing ItMecheri Srinivasan Ganesh100% (1)
- Natural Gas Filters PDFDocument10 pagesNatural Gas Filters PDFKirthiga RamaswamyNo ratings yet
- Variance Analysis Lecture NotesDocument5 pagesVariance Analysis Lecture NotesIena IenaNo ratings yet
- THERMOFLUX PEELING 25 KWDocument48 pagesTHERMOFLUX PEELING 25 KWsorin.agapeNo ratings yet
- Passive MovementDocument16 pagesPassive Movementjetindar33% (3)
- JLG 30am JLG Vertical Lifts Parts ManDocument188 pagesJLG 30am JLG Vertical Lifts Parts ManSebastian GeraciNo ratings yet
- Effective MicroorganismsDocument8 pagesEffective MicroorganismswawahalimNo ratings yet
- Robina Farms Cebu v. VillaDocument9 pagesRobina Farms Cebu v. VillaAnnieNo ratings yet
- Worksafe Bulletin: Carbon Monoxide Exposure During Film ShootsDocument3 pagesWorksafe Bulletin: Carbon Monoxide Exposure During Film ShootsBuddho BuddhaNo ratings yet
- CAFE Request Letter V2Document2 pagesCAFE Request Letter V2CTV CalgaryNo ratings yet
- MODULE 7-11 Notes PrefiDocument7 pagesMODULE 7-11 Notes PrefiPASCUAL, ALJON R.No ratings yet
- Bai Tap Tieng Anh Sach Thi Diem Theo Tung Unit Lop 8 (Co Dap An Chi Tiet)Document122 pagesBai Tap Tieng Anh Sach Thi Diem Theo Tung Unit Lop 8 (Co Dap An Chi Tiet)Yuko YukoNo ratings yet
- Cyclone and Bangladesh A Historical and Environmental Overview From 1582 To 2020Document21 pagesCyclone and Bangladesh A Historical and Environmental Overview From 1582 To 2020Ibnath Nabiha/MF/BRACNo ratings yet
- This Is Us S01E02 HDTV x264-KILLERSDocument31 pagesThis Is Us S01E02 HDTV x264-KILLERSMustafa ErkizNo ratings yet
- Metal Replacement Forum 2014Document287 pagesMetal Replacement Forum 2014eitan-dalia4971100% (1)
- Helical Coil Heat Exchanger Without Agitation (Batch and Continuous) Mas GitoDocument104 pagesHelical Coil Heat Exchanger Without Agitation (Batch and Continuous) Mas GitosehonoNo ratings yet
- Preheat Temperature Table For Different Materials in WeldingDocument1 pagePreheat Temperature Table For Different Materials in WeldingRakesh Soni0% (1)
- Department of Education: Republic of The PhilippinesDocument1 pageDepartment of Education: Republic of The Philippineseric manuevoNo ratings yet
- Nice 7000Document237 pagesNice 7000shahzad farooq100% (1)
- Preparation and Benefits of Electrical Load ScheduleDocument7 pagesPreparation and Benefits of Electrical Load ScheduleMila GamidoNo ratings yet
- Easi-Flo Le: Water HeaterDocument28 pagesEasi-Flo Le: Water HeaterMarino LucasNo ratings yet
- PC 1 FlowAssurance PDFDocument21 pagesPC 1 FlowAssurance PDFAlvaro VelardeNo ratings yet
- PPDDocument347 pagesPPDweilinmdNo ratings yet
- Taste and SmellDocument22 pagesTaste and SmellabdirizakNo ratings yet
- Collaborative Robots Industry Group 1Document13 pagesCollaborative Robots Industry Group 1SoorajKrishnanNo ratings yet
- 合并PDFDocument3 pages合并PDFBozhaoNo ratings yet
- Newly Opened - Untamed Chef RestaurantDocument2 pagesNewly Opened - Untamed Chef RestaurantPR.comNo ratings yet
- Afa LT106Document2 pagesAfa LT106Pedro HerreraNo ratings yet