Professional Documents
Culture Documents
SC CO2andSSC HICMPDec14
Uploaded by
SAUGAT DUTTAOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
SC CO2andSSC HICMPDec14
Uploaded by
SAUGAT DUTTACopyright:
Available Formats
See discussions, stats, and author profiles for this publication at: https://www.researchgate.
net/publication/289574645
Should supercritical CO2 pipelines comply with ANSI/ NACE MR0175/ISO
15156?
Article in Materials Performance · December 2014
CITATIONS READS
3 1,135
1 author:
Bruce Craig
Stress Engineering Services, Inc.
69 PUBLICATIONS 816 CITATIONS
SEE PROFILE
All content following this page was uploaded by Bruce Craig on 08 May 2016.
The user has requested enhancement of the downloaded file.
MATERIALS SELECTION & DESIGN
Should Supercritical CO2
Pipelines Comply With ANSI/
NACE MR0175/ISO 15156?
B. Craig, FNACE, Stress Engineering Recent developments associated with economics for enhanced oil recovery, have
Services, Denver, Colorado carbon capture and storage have sig- significantly increased the number of
nificantly increased the use of carbon projects worldwide that have constructed
steel pipelines for the transport of su- carbon steel (CS) pipelines for transport of
supercritical CO 2. The long, successful
percritical carbon dioxide (CO2). These
history of utilizing bare CS pipelines for this
pipelines are not specifically covered
transport is simply due to the dehydration
by ANSI/NACE MR0175/ISO 15156, of CO 2 to levels that do not permit a
which applies to oil and gas produc- separate liquid water phase to be present.
tion; however, it is prudent to consider However, regardless of how well pipelines
the possible risks of sulfide stress are designed and operated, there is always
cracking and hydrogen-induced crack the potential for water to be present at
ing that can arise in the presence of a some time during the life of the pipeline.
I
liquid water phase. This raises the question of whether sul-
fide stress cracking (SSC) and hydrogen-
induced cracking (HIC), as described by
In 2008, leaders of the Group of Eight NACE International Standard ANSI/NACE/
(G8), a forum of governments from eight MR0175/ISO 15156,1 should be considered
industrialized countries, committed to in the design and construction of super-
broad deployment of carbon capture and critical CO2 pipelines. This is especially rel-
storage (CCS) by 2020 and recommended evant where the CO2 source is gas contain-
the launch of 20 large-scale CCS demonstra- ing hydrogen sulfide (H2S) that is recycled
tion projects by 2010. Since 2008, govern- from gas plants.
ments have committed $26 billion in
funding for large-scale demonstration CCS Industry Standards
projects. Although progress has been made and Requirements
since 2008, the 2010 target was not achieved Even though supercritical CO2 pipelines
for a variety of reasons. To date, there is no have been in service in the United States for
international agreement on a global more than 35 years, there are few standards
response to climate change. A charge on that apply to their design and construction.
carbon emissions, and as-yet-to-be-deter- Supercritical CO 2 pipelines are designed
mined revenue-generating uses for carbon and constructed in accordance with ASME
dioxide (CO2) will be required to make many B31.4,2 which specifically covers CO2 pipe-
of these projects economical. Currently, lines; however, there is no mention of the
only enhanced oil recovery projects have presence of H2S in the CO2 or any require-
positive economics. Yet the continuing ments to consider the potential for crack-
drive for CCS, along with the positive ing from H2S.
2 DECEMBER 2014 MATERIALS PERFORMANCE NACE INTERNATIONAL: VOL. 53, NO. 12
DNV RP J202 3 states, “Pipelines with
fluids containing hydrogen sulfide shall be
evaluated for sour service according to ISO
15156.” Strictly speaking, ANSI/NACE
MR0175/ISO 15156 does not apply to super-
critical CO2 pipelines or facilities since that
standard is specific to oil and gas develop-
ment.
Operating and
Upset Conditions in
Supercritical CO2
Typical sources of CO2 for enhanced oil
recovery come from tail gas off of gas
plants, while CCS projects are largely
aimed at sources from coal-fired power
plants and cement plants. In some cases,
actual CO2 reservoirs are produced in loca-
tions such as Jackson Dome, Mississippi,
and the Four Corners area of Colorado. The
CO 2 can contain a variety of impurities
depending on their source, including meth-
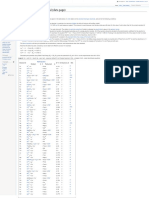
ane (CH4), hydrogen gas (H2), nitrogen gas FIGURE 1 Effect of temperature and CO2 pressure on pH of water in supercritical CO2.
(N2), H2S, nitrogen oxides (NOx), and sulfur
oxides (SOx).
The critical pressure for pure CO 2 is to determine whether the systems would restricted to a few days or even weeks, and
1,088 psi (7.5 MPa) and the critical temper- contain free liquid water. For example, if not months.
ature is 88 °F (31 °C). However, it is com- hydrates form in preference to liquid water,
mon for CO2 pipelines in the United States then corrosion—and thus HIC—might not Risk of SSC and HIC in
to operate at 2,220 psi (15.3 MPa) and be a significant problem. The phases that Supercritical CO2
~100 °F (38 °C). will form under normal pipeline operating There are no studies or data regarding
The design and operation of supercriti- conditions and for upsets can be modeled the potential for HIC of line pipe or plate
cal CO2 pipelines requires consideration of using the CSMGem program.4 According to steel when exposed to supercritical CO 2
several important mechanisms beyond the Sloan,4 if the temperature of the CO2 pipe- that contains H 2S. It is uncertain at this
common hydraulic and mechanical proper- line is 6.5 °F lower than the temperature time whether HIC or SSC would even occur
ties. These are water content of the fluid calculated by CSMGem for hydrate forma- under these conditions, and whether or not
( from a corrosion and hydrate perspective) tion (or even lower than 6.5 °F), it can be the H 2S cracking thresholds used for gas
and the risk of running ductile fractures. assumed that hydrates form immediately. If and oil service would be the same for
While the latter factor is very important to hydrates form but no liquid water is pres- supercritical CO2.
the design of supercritical CO2 pipelines, it ent, then corrosion will not occur and HIC Just as for corrosion in gaseous CO2, the
is not addressed in this article. is unlikely. risk of HIC in supercritical CO2 will depend
It is well known that supercritical CO2 is Once pipeline conditions are estab- on the presence of liquid water. The pH of
not corrosive unless a free liquid water lished and water is not a normal constitu- free water under supercritical CO2 condi-
phase is present in the pipeline. This can ent, the risk of HIC is limited by the fre- tions can be quite low. Choi and Nes̆ić5 found
occur for various reasons, such as hydrotest quency and duration of upsets that produce that the pH would be ~3.0 to 3.2 under
water that remains in the pipeline and liquid water and the time-of-wetness on supercritical conditions. Figure 1 shows the
upset of the dehydration system. Assuming the pipe walls. If possible, time-of-wetness relationship between CO2 pressure, temper-
liquid water is present, there is a risk of should be limited, since the long-term pres- ature, and the resulting pH from their work.
hydrate formation. While it is beyond the ence of water will cause corrosion and the These investigators found corrosion
scope of this article, it is important to con- risk that hydrates will form, which could rates of 18 to 20 mm/y (710 to 788 mpy) in
sider the likely phases present in CO2-rich hamper operations. It can only be specu- the liquid water phase. In the presence of
systems during operation and shut downs lated that time-of-wetness is likely to be liquid water, SSC of steel pipelines is a risk
NACE INTERNATIONAL: VOL. 53, NO. 12 MATERIALS PERFORMANCE DECEMBER 2014 3
MATERIALS SELECTION & DESIGN
described by ANSI/NACE MR0175/ISO cially when there is an incubation period of References
15156. When H 2S is present in the CO 2 hours or days before achieving sufficient 1 ANSI/NACE MR0175/ISO 15156, “Petroleum
stream, the rapid, catastrophic nature of hydrogen absorption to initiate cracks. and natural gas industries—Materials for use
SSC makes consideration essential during in H2S-containing environments in oil and
supercritical CO2 pipeline design. Discussion and Conclusions gas production” (Houston, TX: NACE Inter-
In contrast, HIC is often a much slower Although currently there are no regula- national, 2009).
and less catastrophic cracking mechanism, tory requirements to design and construct 2 ASME B31.4, “Pipeline Transportation Sys-
which also requires some consideration. supercritical CO 2 pipelines to resist SSC tems for Liquids and Slurries” (New York, NY:
McIntyre6 presented over 25 case histories and HIC if they contain some H 2 S, it ASME International, 2012).
from the Middle East of HIC in pipelines appears prudent as a minimum safeguard 3 DNV RP J202, “Design And Operation of CO2
and piping covering years of service. He to mitigate the risk of SSC. However, the Pipeline” (Høvik, Norway: DNV, 2010).
reported HIC crack growth rates ranging risk of HIC is more difficult to assess in 4 E. Dendy Sloan, C.A. Koh, Clathrate Hydrates
from 0.0 to 0.036 in/y (0.9 mm/y) with one these systems. The corrosion rate from of Natural Gases, 3rd ed. (Boca Raton, FL:
exception where failure occurred in 10 days supercritical CO2 when a free water phase CRC Press, 2008).
(~4 in/y [102 mm/y]). This premature fail- is present can be faster than HIC crack 5 Y. Choi, S. Nes̆ić, “Corrosion Behavior of Car-
ure was in the highest H2S partial pressure growth rates, and hydrates can form so bon Steel in Supercritical CO2—Water Envi-
(84 kPa) and lowest pH (3.7) of all the cases quickly that HIC is not likely. Moreover, if ronments,” CORROSION 2009, paper no.
09256 (Houston, TX: NACE, 2009).
reported. the presence of a water phase is due to
In laboratory studies, the crack initia- short duration upsets, the dry supercritical 6 D.R. McIntyre, “Step-Wise Cracking Growth
Rates from Service Incidents,” Proc. 8th Mid-
tion rate and growth rates are typically CO2 entering the pipeline can be expected
dle East Corrosion Conference, held May
much faster than in actual service for vari- to remove any liquid water, thereby drying
18-20, 1998 (Houston, TX: NACE, 1998), pp.
ous reasons, such as only one-sided charg- the pipeline and eliminating the free water
594-604.
ing in service compared to multisided phase necessary for HIC to proceed.
7 ANSI/NACE Standard Test Method TM0284-
charging in laboratory specimens (NACE The frequency and duration of upsets
2011, “Evaluation of Pipeline and Pressure
standard test method TM0284).7 Also, com- that permit water to be present in the pipe- Vessel Steels for Resistance to Hydrogen-
pared to small laboratory samples, there line must be considered in the decision to Induced Cracking” (Houston, TX: NACE,
are much larger volumes of metal in a pipe- require HIC resistance. At the expected low 2011).
line where hydrogen can accumulate before pH of water in contact with supercritical 8 J. Gonzales R. Ramirez, J.M. Hallen, R.A. Guz-
crack initiation. Gonzalez, et al.8 found a CO2, the hydrogen absorption and perme- man, “Hydrogen-Induced Crack Growth Rate
crack growth rate of ~0.09 in/d (2.3 mm/d) ation could be significant. (TM0284 tests in in Steel Plates Exposed to Sour Environ-
in test samples exposed to a solution satu- Solution A represent a similar pH [~2.7]; ments,” Corrosion 53 (1997): p. 935.
rated with H2S. This rate was before crack however, the test solution only contains 9 M. Cayard, C. Joia, P. Bezerra, F. Assuncao,
linkage, which is an important factor in the H 2S—no CO 2.) While it is less aggressive “Fracture Toughness and Mechanical Prop-
risk of HIC. Many companies detect HIC than H 2S in enhancing the absorption of erties of C-Mn Steels Exposed To Wet H2S
early in the process of crack advance but hydrogen in steels, CO 2 still contributes Environments,” CORROSION/99, paper no.
prior to linkage and allow the pipeline or hydrogen to the total amount diffusing into 384 (Houston, TX: NACE, 1999).
vessel to operate under some limitations the steel. In addition, since hydrogen is BRUCE CRAIG, FNACE, is the subject matter
until linkage occurs and the component trapped in pipeline steels with little egress expert at Stress Engineering Services, 13800
Westfair East Dr., Bldg. 3, Houston, TX 77041,
becomes unsafe to operate. after the water is removed, periodic upsets e-mail: bruce.craig@stress.com. He has been
Cayard, et al.9 reported the crack length will cause hydrogen to accumulate at traps, involved with the evaluation of corrosive oil
ratio (% CLR) for two steels, from which the and potentially lead to HIC crack initiation and gas wells and the transportation of these
corrosive fluids for projects ranging from
crack growth rate can be approximated. at a later time. Therefore, until hydrogen Mobile Bay to the deep water Gulf of Mexico
For four days of exposure (the required permeation studies can be made using and Asia and the Middle East for more than
time frame for HIC testing per NACE supercritical CO2 with H2S and these results 30 years. In addition he has consulted on many
projects around the world, including modeling
TM0284), the crack growth rate was essen- are coupled with HIC tests in the same corrosion from CO2 in wet gas flow lines and
tially zero for one steel and 0.18 in/d (4.6 environment, it is impossible to be confi- pipelines. He has consulted on numerous CO2
mm/d) for another. After 30 days the rates dent that HIC is not a potential threat for pipelines used to transport supercritical CO 2
and is an expert in sulfide stress cracking. He
were 0.05 and 0.07 in/d (1.3 and 1.8 mm/d), supercritical CO 2 pipelines. Until labora- has a Ph.D. in metallurgical engineering and
respectively. tory testing has been performed to estab- has published more than 90 papers and seven
Thus, it can be seen that the crack lish the level of risk for HIC in supercritical books. A member of NACE International for
30 years, Craig is a Senior Research Fellow at
growth rate from HIC is quite variable and CO2, it is prudent to require these pipeline the Institute for Corrosion and Multiphase
with few exceptions, is relatively slow, espe- steels to be HIC resistant. Flow, Ohio University.
4 DECEMBER 2014 MATERIALS PERFORMANCE NACE INTERNATIONAL: VOL. 53, NO. 12
View publication stats
You might also like
- Design of Carbon Capture and Sequestration CCS WellsDocument12 pagesDesign of Carbon Capture and Sequestration CCS Wellsartdanielz09No ratings yet
- Carbon Dioxide Pipeline-A Preliminary Review of Design and RisksDocument6 pagesCarbon Dioxide Pipeline-A Preliminary Review of Design and RisksbobcyliaoNo ratings yet
- 1 s2.0 S1876610217318647 MainDocument17 pages1 s2.0 S1876610217318647 MainMoj TabaNo ratings yet
- CO2 HazardDocument5 pagesCO2 HazardAndi SuntoroNo ratings yet
- Fracture Propagation of Co2 PipelinesDocument10 pagesFracture Propagation of Co2 Pipelinesjemfus10100% (1)
- 2019-09 DNV Safely Re-Using Infrastructure For CO2 Transport and StorageDocument5 pages2019-09 DNV Safely Re-Using Infrastructure For CO2 Transport and StoragehefflingerNo ratings yet
- Enhance Energy Design DetailsDocument43 pagesEnhance Energy Design DetailsFredrick MartinNo ratings yet
- SPE 168294 Coiled Tubing Material Selection For Velocity Strings in Sour Brine ServiceDocument13 pagesSPE 168294 Coiled Tubing Material Selection For Velocity Strings in Sour Brine ServiceJamshed SoomroNo ratings yet
- 6 Pipeline Technology Conference 2011Document13 pages6 Pipeline Technology Conference 2011Anonymous 6LwW4qi6TiNo ratings yet
- 1 s2.0 S1350630721000534 MainDocument15 pages1 s2.0 S1350630721000534 Mainjolugoto1991No ratings yet
- 1 s2.0 S0360319920304262 MainDocument6 pages1 s2.0 S0360319920304262 MainBharatSuryaNo ratings yet
- Transport of CO2Document16 pagesTransport of CO2Archie SmileyNo ratings yet
- Chemical Engineering Journal: Yafei Guo, Chuanwen Zhao, Changhai Li, Ye WuDocument9 pagesChemical Engineering Journal: Yafei Guo, Chuanwen Zhao, Changhai Li, Ye WuFarah Talib Al-sudaniNo ratings yet
- Transport of CO: Coordinating Lead Authors Lead AuthorsDocument16 pagesTransport of CO: Coordinating Lead Authors Lead AuthorsGaurav MishraNo ratings yet
- CO Pipelines Material and Safety ConsiderationsDocument7 pagesCO Pipelines Material and Safety Considerationsred patriotNo ratings yet
- 2015 Effect of H2S On The Corrosion Behavior of Pipeline Steels in Supercritical and Liquid Co2 EnvironmentsDocument12 pages2015 Effect of H2S On The Corrosion Behavior of Pipeline Steels in Supercritical and Liquid Co2 EnvironmentsRuben CuamatziNo ratings yet
- Carbon Dioxide Enhanced Oil Recovery Injection Operations TechnologiesDocument8 pagesCarbon Dioxide Enhanced Oil Recovery Injection Operations TechnologiesSaeid RajabiNo ratings yet
- Carbon Capture and Storage (CCS) : January 2015Document13 pagesCarbon Capture and Storage (CCS) : January 2015chirag sabhayaNo ratings yet
- Iatmi22 080Document6 pagesIatmi22 080artdanielz09No ratings yet
- SPE 161207 Managing The Corrosion Impact of Dense Phase Co Injection For An EOR PurposeDocument11 pagesSPE 161207 Managing The Corrosion Impact of Dense Phase Co Injection For An EOR PurposeTurqay İsgəndərliNo ratings yet
- Corrosion Control in CO Enhanced Oil Recovery From A Perspective of Multiphase FluidsDocument16 pagesCorrosion Control in CO Enhanced Oil Recovery From A Perspective of Multiphase Fluidshoss mosafaNo ratings yet
- Acid Gas Injection in USDocument21 pagesAcid Gas Injection in USicelemon_zhouNo ratings yet
- Corrosion Behaviour of X65 Carbon Steel in Supercritical-CO2containing H2O and O2in Carbon Capture and Storage (CCS) TechnologyDocument11 pagesCorrosion Behaviour of X65 Carbon Steel in Supercritical-CO2containing H2O and O2in Carbon Capture and Storage (CCS) TechnologyLuis LozadaNo ratings yet
- Overcoming of CO2 Injector Well Design & Completion Challenges in Carbonate ReservoirDocument15 pagesOvercoming of CO2 Injector Well Design & Completion Challenges in Carbonate Reservoirartdanielz09No ratings yet
- 1 s2.0 S095006182203402X MainDocument15 pages1 s2.0 S095006182203402X Mainaashima sharmaNo ratings yet
- 1 s2.0 S221298202200110X MainDocument13 pages1 s2.0 S221298202200110X MainHappy LightNo ratings yet
- CO2 Pipeline Design A ReviewDocument29 pagesCO2 Pipeline Design A ReviewAmmarul NafikNo ratings yet
- Effect of Small Amount of H2S On The Corrosion BehaviorDocument43 pagesEffect of Small Amount of H2S On The Corrosion BehaviorRagerishcire KanaalaqNo ratings yet
- Selection of Materials For High Pressure CO2 Transport: Technical Knowledge Published PapersDocument15 pagesSelection of Materials For High Pressure CO2 Transport: Technical Knowledge Published PapersJohn DNo ratings yet
- (2012) Discussion of The CO2 Corrosion MechanismDocument12 pages(2012) Discussion of The CO2 Corrosion MechanismMedaculoNo ratings yet
- Spe 207354 Ms PublishedDocument21 pagesSpe 207354 Ms Publishedsawsan311898No ratings yet
- P.7 KaufmannDocument28 pagesP.7 KaufmannMuhammad Zeeshan WasiNo ratings yet
- Experimental Development of Calcium Looping CarbonDocument27 pagesExperimental Development of Calcium Looping CarbonRobson Rocha OliveiraNo ratings yet
- Paper-106817 SPEDocument16 pagesPaper-106817 SPEEdison Javier Rodríguez AmezquitaNo ratings yet
- 9 CorrosDocument25 pages9 CorrosFrancisco Beltran100% (1)
- Decompression Characteristics of CO2 Pipelines Following RuptureDocument11 pagesDecompression Characteristics of CO2 Pipelines Following RuptureMohdFarid RahmatSamNo ratings yet
- CCUS Chap - 6 030521Document18 pagesCCUS Chap - 6 030521Ali AltowilibNo ratings yet
- Review Article Well Integrity Evaluation During CO Storage and Enhanced Gas RecoveryDocument8 pagesReview Article Well Integrity Evaluation During CO Storage and Enhanced Gas RecoveryPetroleum EngineerNo ratings yet
- Cement Degradation in CO2 Storage Sites A Review oDocument12 pagesCement Degradation in CO2 Storage Sites A Review oEdinson Diaz RojasNo ratings yet
- Omae2012 83321Document9 pagesOmae2012 83321Vinicius Cantarino CurcinoNo ratings yet
- The Use of Corrosion Inhibitors in Oil and GasDocument7 pagesThe Use of Corrosion Inhibitors in Oil and Gasdodofan2000No ratings yet
- Techno-Economic Analyses of CO2 Liquefaction - Impact of Product Pressure and ImpuritiesDocument15 pagesTechno-Economic Analyses of CO2 Liquefaction - Impact of Product Pressure and Impuritieskglorstad100% (1)
- Hazards Associated With CCS 1Document10 pagesHazards Associated With CCS 1Yahyah NahabooNo ratings yet
- CCSDocument22 pagesCCSkingsley kaputoNo ratings yet
- Research On The Steel For Oil and Gas Pipelines in Sour EnvironmentDocument5 pagesResearch On The Steel For Oil and Gas Pipelines in Sour EnvironmentHomam MohammadNo ratings yet
- DesignEnggPaper Hydrogen1998Document5 pagesDesignEnggPaper Hydrogen1998omiitgNo ratings yet
- Design For Continuous PCC ProducitonDocument11 pagesDesign For Continuous PCC Producitongabriela rahayuNo ratings yet
- SPE 109294 ACO - Rich Gas Well Test and AnalysesDocument10 pagesSPE 109294 ACO - Rich Gas Well Test and AnalysesAnonymous VNu3ODGavNo ratings yet
- Chem: Hassan Farag HassanDocument18 pagesChem: Hassan Farag Hassanhasan100% (2)
- 10 1016@j Ijggc 2019 04 016Document23 pages10 1016@j Ijggc 2019 04 016kiingkangNo ratings yet
- 2021 02 ROSEN PaperDocument13 pages2021 02 ROSEN Papermostafa shahrabiNo ratings yet
- A Review of Cement Testing Apparatus and Methods Under CO2 Environment and Their Impact On Well Integrity Prediction - Where Do We StandDocument24 pagesA Review of Cement Testing Apparatus and Methods Under CO2 Environment and Their Impact On Well Integrity Prediction - Where Do We StandXuning WuNo ratings yet
- 2010 Fracture Control Strategy For Conversion of O&G Pipelines To CO2Document12 pages2010 Fracture Control Strategy For Conversion of O&G Pipelines To CO2hefflingerNo ratings yet
- Corrosion in The Oil and Gas Industry-An Increasing Challenge For MaterialsDocument10 pagesCorrosion in The Oil and Gas Industry-An Increasing Challenge For Materialswidjai10No ratings yet
- Technology Scouting Carbon Capture From Todays To Novel TechnologiesDocument11 pagesTechnology Scouting Carbon Capture From Todays To Novel TechnologiesTarek Ahmed AbdelhadyNo ratings yet
- Carbon Capture StorageDocument9 pagesCarbon Capture StorageTS WongNo ratings yet
- Recent Aspects of Oil and Gas Internal Pipeline Corrosion ControlDocument25 pagesRecent Aspects of Oil and Gas Internal Pipeline Corrosion ControlaseNo ratings yet
- Corrosion-Resistant Alloy Testing and SelectionDocument16 pagesCorrosion-Resistant Alloy Testing and SelectionrenadimNo ratings yet
- Cutting-Edge Technology for Carbon Capture, Utilization, and StorageFrom EverandCutting-Edge Technology for Carbon Capture, Utilization, and StorageKarine Ballerat-BusserollesNo ratings yet
- HDD-LC-0001 Rev 0Document24 pagesHDD-LC-0001 Rev 0SAUGAT DUTTANo ratings yet
- Details of Mattress Break - Depth Vs Rows & Columns (Rev 002 - 17032024) - 1Document9 pagesDetails of Mattress Break - Depth Vs Rows & Columns (Rev 002 - 17032024) - 1SAUGAT DUTTANo ratings yet
- Asmepvp2018 84458 FinalDocument8 pagesAsmepvp2018 84458 FinalMark Angello SibayanNo ratings yet
- Urea PlantDocument5 pagesUrea PlantWael MansourNo ratings yet
- DNV Recommended Practice - Design and Operation of CO2 Pipelines 2011 PDFDocument8 pagesDNV Recommended Practice - Design and Operation of CO2 Pipelines 2011 PDFYvesfNo ratings yet
- Iso-648 Laboratory Glassware - Single-Volume PipettesDocument18 pagesIso-648 Laboratory Glassware - Single-Volume PipettesDawn HaneyNo ratings yet
- Supply Reference Details of The Supplies Made in Last Ten Years Duly Filled in Format C.Document29 pagesSupply Reference Details of The Supplies Made in Last Ten Years Duly Filled in Format C.SAUGAT DUTTANo ratings yet
- Amethodformeasuringhydrodynamicforcecoefficientsappliedtoanarticulatedconcretemattress PDFDocument13 pagesAmethodformeasuringhydrodynamicforcecoefficientsappliedtoanarticulatedconcretemattress PDFSAUGAT DUTTANo ratings yet
- Test Report - 37Document2 pagesTest Report - 37SAUGAT DUTTANo ratings yet
- Essay Arush Dutta - Hardest ChallengeDocument1 pageEssay Arush Dutta - Hardest ChallengeSAUGAT DUTTANo ratings yet
- 6-71-0014 Rev 5Document16 pages6-71-0014 Rev 5SAUGAT DUTTANo ratings yet
- DocumentDocument1 pageDocumentSAUGAT DUTTANo ratings yet
- Cairn-6682 - Complete DocumentDocument54 pagesCairn-6682 - Complete DocumentSAUGAT DUTTANo ratings yet
- R.D Rajpal School Assignment For Revision Class - Vii Topic: Articles A) Fill in The Blanks With Appropriate ArticlesDocument2 pagesR.D Rajpal School Assignment For Revision Class - Vii Topic: Articles A) Fill in The Blanks With Appropriate ArticlesSAUGAT DUTTANo ratings yet
- Essay Arush Dutta - Hardest ChallengeDocument1 pageEssay Arush Dutta - Hardest ChallengeSAUGAT DUTTANo ratings yet
- Eil Pipeline Engineering Comments On SNY ValvesDocument1 pageEil Pipeline Engineering Comments On SNY ValvesSAUGAT DUTTANo ratings yet
- DocumentDocument1 pageDocumentSAUGAT DUTTANo ratings yet
- Poem - Lady ClareDocument2 pagesPoem - Lady ClareSAUGAT DUTTANo ratings yet
- Adc B TTDocument1 pageAdc B TTSAUGAT DUTTANo ratings yet
- Eil Pipeline Engineering Comments On SNY ValvesDocument1 pageEil Pipeline Engineering Comments On SNY ValvesSAUGAT DUTTANo ratings yet
- DocumentDocument1 pageDocumentSAUGAT DUTTANo ratings yet
- Essay Arush Dutta - Hardest ChallengeDocument1 pageEssay Arush Dutta - Hardest ChallengeSAUGAT DUTTANo ratings yet
- Identify The Following As Phrases, Clauses or Complete SentenceDocument2 pagesIdentify The Following As Phrases, Clauses or Complete SentenceSAUGAT DUTTANo ratings yet
- Principal R. D. Rajpal SchoolDocument1 pagePrincipal R. D. Rajpal SchoolSAUGAT DUTTANo ratings yet
- R.D Rajpal School Assignment For Revision Class - Vii Topic: Articles A) Fill in The Blanks With Appropriate ArticlesDocument2 pagesR.D Rajpal School Assignment For Revision Class - Vii Topic: Articles A) Fill in The Blanks With Appropriate ArticlesSAUGAT DUTTANo ratings yet
- Principal R. D. Rajpal SchoolDocument1 pagePrincipal R. D. Rajpal SchoolSAUGAT DUTTANo ratings yet
- R.D.Rajpal School Academic Planner (2019-20) Class Vii April & MayDocument19 pagesR.D.Rajpal School Academic Planner (2019-20) Class Vii April & MaySAUGAT DUTTANo ratings yet
- 1569855365405Document1 page1569855365405SAUGAT DUTTANo ratings yet
- Poem - Lady ClareDocument2 pagesPoem - Lady ClareSAUGAT DUTTANo ratings yet
- Synthetic Detergents 100 Years of HistoryDocument16 pagesSynthetic Detergents 100 Years of HistoryJayantha TennakoonNo ratings yet
- MSDS R6Document3 pagesMSDS R6jafarptrNo ratings yet
- O LVL Chemistry West Spring Sec Prelim 2020iDocument38 pagesO LVL Chemistry West Spring Sec Prelim 2020iMichelle LimNo ratings yet
- Assignment 5: Engineering Utilities IiDocument4 pagesAssignment 5: Engineering Utilities IiRex SabersonNo ratings yet
- HPLC Analysis of Mono-And Disaccharides in Food Products: October 2013Document6 pagesHPLC Analysis of Mono-And Disaccharides in Food Products: October 2013Long PhiNo ratings yet
- En 13835Document12 pagesEn 13835Aditya PratapNo ratings yet
- We Are Intechopen, The World'S Leading Publisher of Open Access Books Built by Scientists, For ScientistsDocument51 pagesWe Are Intechopen, The World'S Leading Publisher of Open Access Books Built by Scientists, For ScientistssantanuaNo ratings yet
- Sikaplan WP 1100-15HLDocument2 pagesSikaplan WP 1100-15HLalparslan GÜRENo ratings yet
- Sikagrout®-114 Ae: Product Data SheetDocument3 pagesSikagrout®-114 Ae: Product Data SheetAlexander Jonas Zach ValdrizNo ratings yet
- Project Report On Bromine Recovery PlantDocument6 pagesProject Report On Bromine Recovery PlantEIRI Board of Consultants and PublishersNo ratings yet
- Peter Atkins Julio de Paula Ron Friedman Physical Chemistry Quanta (0766-0816)Document51 pagesPeter Atkins Julio de Paula Ron Friedman Physical Chemistry Quanta (0766-0816)Administracion OTIC IVICNo ratings yet
- Chapter 4Document51 pagesChapter 4Hà Nguyễn Thị NgọcNo ratings yet
- Sorez-205 Sell Sheet VF PDFDocument2 pagesSorez-205 Sell Sheet VF PDFmaheshNo ratings yet
- Mohamed Abdel-Hameed C.V 2020Document3 pagesMohamed Abdel-Hameed C.V 2020Hatem HusseinNo ratings yet
- Suto S130-S132 en 23-1 PDFDocument5 pagesSuto S130-S132 en 23-1 PDFVikas PatidarNo ratings yet
- CRH Cellular Respiration (Principles) - Measure Energy Consumption During Exercise Lab ManualDocument6 pagesCRH Cellular Respiration (Principles) - Measure Energy Consumption During Exercise Lab ManualVanesha AnesNo ratings yet
- Sans 5839Document9 pagesSans 5839Sergio VianaNo ratings yet
- ASTM F639-09 Standard Specification For Polyethylene Plastics For Medical ApplicationsDocument3 pagesASTM F639-09 Standard Specification For Polyethylene Plastics For Medical ApplicationsJoãoNo ratings yet
- Evaluation of Quality of A Drug Product: Prepared and Presented byDocument22 pagesEvaluation of Quality of A Drug Product: Prepared and Presented byAlekhya GuntupalliNo ratings yet
- Spectrophotometric Determination of IronDocument3 pagesSpectrophotometric Determination of IronDozdi93% (14)
- QQ-A-225G - GEN - Aluminum Alloy, Rolled, CD, CFDocument21 pagesQQ-A-225G - GEN - Aluminum Alloy, Rolled, CD, CFthomasNo ratings yet
- Oracal 970 RA Premium Special EffectDocument2 pagesOracal 970 RA Premium Special EffectIonut ValentinNo ratings yet
- Olive Oil and Clove Oil Based Nanoemulsion For Topical Delivery of Terbinafine Hydrochloride in Vitro and Ex Vivo EvaluationDocument14 pagesOlive Oil and Clove Oil Based Nanoemulsion For Topical Delivery of Terbinafine Hydrochloride in Vitro and Ex Vivo EvaluationRaghavendra NaveenNo ratings yet
- Barachina Ecm3Document9 pagesBarachina Ecm3ALDRIAN BARACHINANo ratings yet
- Activity 4-Atomic CrystalsDocument2 pagesActivity 4-Atomic CrystalsVan LeronNo ratings yet
- Standard Electrode Potential (Data Page) - WikipediaDocument5 pagesStandard Electrode Potential (Data Page) - WikipediashahinNo ratings yet
- TPR2Document10 pagesTPR2WILFREDO ROMAN PAUCARNo ratings yet
- W4L1 Combustion ReactionsDocument30 pagesW4L1 Combustion ReactionsYahia MetwalliNo ratings yet
- Chemical Reactions and Equations With Answers Set 1Document6 pagesChemical Reactions and Equations With Answers Set 1Anjali JhaNo ratings yet
- Nitro Benzene Preparation, Laboratory & Industrial, Uses and ApplicationsDocument11 pagesNitro Benzene Preparation, Laboratory & Industrial, Uses and Applicationsusman_uet0881% (16)