Professional Documents
Culture Documents
Effect - of - Fetal - and - Child - Health Hypertention Cardiovask Risk Kidney
Uploaded by
Nia Prajnya SyailendraOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Effect - of - Fetal - and - Child - Health Hypertention Cardiovask Risk Kidney
Uploaded by
Nia Prajnya SyailendraCopyright:
Available Formats
Series
Global Kidney Disease 4
Effect of fetal and child health on kidney development and
long-term risk of hypertension and kidney disease
Valerie A Luyckx, John F Bertram, Barry M Brenner, Caroline Fall, Wendy E Hoy, Susan E Ozanne, Bjorn E Vikse
Developmental programming of non-communicable diseases is now an established paradigm. With respect to Lancet 2013; 382: 273–83
hypertension and chronic kidney disease, adverse events experienced in utero can affect development of the fetal Published Online
kidney and reduce final nephron number. Low birthweight and prematurity are the most consistent clinical surrogates May 31, 2013
http://dx.doi.org/10.1016/
for a low nephron number and are associated with increased risk of hypertension, proteinuria, and kidney disease in
S0140-6736(13)60311-6
later life. Rapid weight gain in childhood or adolescence further compounds these risks. Low birthweight, prematurity,
This is the fourth in a Series of
and rapid childhood weight gain should alert clinicians to an individual’s lifelong risk of hypertension and kidney six papers about global
disease, prompting education to minimise additional risk factors and ensuring follow-up. Birthweight and prematurity kidney disease
are affected substantially by maternal nutrition and health during pregnancy. Optimisation of maternal health and Division of Nephrology,
early childhood nutrition could, therefore, attenuate this programming cycle and reduce the global burden of University of Alberta,
hypertension and kidney disease in the future. Edmonton, AB, Canada
(V A Luyckx MBBCh);
Department of Anatomy and
Introduction Increasingly, the important contribution of fetal Developmental Biology,
Hypertension is now the leading risk factor for the global and early childhood development to growth in non- Monash University, Melbourne,
disease burden and it is a major cause and result of communicable disease is being recognised.3,4 Epi- VIC, Australia
(Prof J F Bertram PhD); Brigham
chronic kidney disease.1 Global deaths from kidney demiological observation suggests a graded risk for and Women’s Hospital, Harvard
disease have risen by 83% since 1990.2 Recognition of the hypertension, type 2 diabetes, cardiovascular disease, Medical School, Boston, MA,
burden of chronic kidney disease, its risk factors, and and chronic kidney disease across the range of fetal and USA (Prof B M Brenner MD);
MRC Lifecourse Epidemiology
implementation of prevention strategies is, therefore, early childhood development.3,4 Acknowledgment of this
Unit, Southampton General
key to saving many lives. paradigm is important because interventions to optimise Hospital, Southampton, UK
fetal and child health as strategies to prevent adult non- (Prof C Fall FRCPCH); Center for
communicable diseases have great potential economic, Chronic Disease, School of
Search strategy and selection criteria Medicine, University of
societal, and individual benefit.4 Many developing coun-
Queensland School of
We searched PubMed for articles published between January, tries carry the dual burdens of undernutrition and Medicine, Brisbane, QLD,
1988, and February, 2013, with the terms “nephron number”, overnutrition, contributing to the vicious cycles of poor Australia
“nephron endowment”, “nephron mass”, “nephrogenesis”, maternal health, suboptimum fetal development, and (Prof W E Hoy FRACP AO);
University of Cambridge
“birth weight”, “low birth weight”, “high birth weight”, unhealthy childhood growth that all augment the risk of
Metabolic Research
“prematurity”, “preterm birth”, “developmental adult disease.5,6 Laboratories, Institute of
programming”, “developmental origins of adult health and
disease”, “catch-up growth”, “growth restriction”, “SGA”, and
Key messages
“IUGR”, with other keywords including “kidney”, “kidney
mass”, “kidney size”, “kidney volume”, “diabetes”, “gestational • Low birthweight and prematurity are risk factors for hypertension, proteinuria, and
diabetes”, “cardiovascular disease”, “obesity”, “human”, chronic kidney disease in later life
“hypertension”, “hypertensive disorders in pregnancy”, • Worldwide, low birthweight and prematurity occur in 15% and 9·6% of livebirths,
“preeclampsia”, “vitamin A deficiency”, “maternal diet”, and respectively, suggesting a high proportion of the world’s children are at risk of
“maternal nutrition”. We also looked at the reference lists of hypertension and kidney disease
existing manuscripts, textbooks, and websites. Furthermore, • Low birthweight and prematurity are associated with a congenital reduction in nephron
we identified data, references, and links by searching the number; in turn, small numbers of nephrons are associated with raised blood pressure
WHO, UNICEF, and Google Scholar websites between and increased susceptibility to kidney disease
January, 2012, and February, 2013, with the keywords “low • High birthweight, particularly as a result of exposure to maternal diabetes in utero, is
birth weight”, “preeclampsia”, “gestational diabetes”, associated with increased risk of proteinuria and kidney disease in later life
“maternal and newborn health”, “nutrition”, and “childhood • Risk of low birthweight and prematurity is affected by maternal nutrition and health
obesity”. We restricted our search to English language before and during pregnancy and by the mother’s own birthweight, indicating the
publications only. We largely included publications from the intergenerational effects of programming
past 5 years but also considered older seminal papers. Some • Upward crossing of percentiles for weight or body-mass index in childhood or
references to experimental data were included when these adolescence is associated with increased risk of high blood pressure, progression of renal
were judged necessary to explain ideas strongly supporting disease, type 2 diabetes, obesity, and cardiovascular disease in later life; these effects can
the pathophysiology but not yet proven in man. be independent of birthweight
www.thelancet.com Vol 382 July 20, 2013 273
Series
Metabolic Science, Cambridge,
UK (S E Ozanne PhD); and Pregnancy
Institute of Medicine,
University of Bergen, and Maternal diabetes Maternal Societal factors Maternal health Genetic polymorphisms
Department of Medicine, or gestational development Poverty, conflict or war, Low protein diet, pre-eclampsia, PAX2, RET, OSR1, ACE,
Haukeland University Hospital, diabetes Low birthweight, teenage pregnancy, hypertension, vitamin A deficiency, iron BMPR1A
Bergen, Norway short stature antenatal care, birth spacing, deficiency, substance abuse, malaria, chronic
(Prof B E Vikse MD) environmental conditions kidney disease, multiple gestation
Childhood/fetal development
Correspondence to: High birthweight
Dr Valerie A Luyckx, Division of
Low nephron number
Nephrology, University of Alberta, Intrauterine growth restriction or Clinical surrogates:
Edmonton, AB, Canada T6G 2S2 low birthweight or prematurity Low birthweight, prematurity, short stature, gestational diabetes
vluyckx@ualberta.ca exposure, ethnic origin, large glomeruli, small kidneys
Catch-up growth
Overweight or obese Hypertension Hyperfiltration Proteinuria
and salt sensitivity ↓ Renal functional reserve
↓ Glomerular filtration rate
Metabolic Ischaemic
Adult
syndrome heart disease
Diabetes Glomerulosclerosis, chronic kidney disease, end-stage renal disease
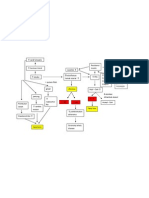
Figure 1: Factors affecting developmental programming of hypertension and kidney disease
Here, we describe how fetal and child health affect whereby low birthweight was expected to be associated
kidney development and risk of disease. We focus with a lower number of nephrons (figure 1).8 Consistent
mainly on human studies but use experimental data with this hypothesis, data from various experimental
when necessary to provide further insight. models confirm the association of low birthweight with
later-life hypertension, mediated, in part, by acquisition
Effect of fetal development on the kidney of fewer nephrons in utero (the pathophysiological
About 25 years ago, Brenner and colleagues7 pro- mechanisms affecting nephrogenesis are reviewed
posed that a congenital (developmentally programmed) elsewhere).9 Similarly, in human beings, low birth-
decrease in nephron number could account for why weight is a risk factor for hypertension and chronic
some individuals are more susceptible to hypertension kidney disease.10
and renal injury than others. A kidney with fewer Low birthweight is a marker of poor fetal growth.
nephrons was postulated to have a diminished filtration Risk factors for low birthweight vary in developed and
surface area, resulting in limitation of sodium excretion developing countries but the global incidence is 15%
leading to raised blood pressure and reduction of a year, suggesting that many children are at risk of
renal adaptive capacity in the setting of injury. This hypertension and kidney disease in later life (figure 2).11–15
hypothesis provided a plausible link between a high In view of the complexity and far-reaching effect of
prevalence of hypertension and renal disease in popu- developmental programming, understanding the most
lations with an increased frequency of low birthweight, proximal origins of hypertension and renal disease risk
is crucial for expansion of public health strategies to
35 Low birthweight (% livebirths) reduce their global effect.4
Preterm birth (% livebirths) Low birthweight is defined universally as a birth-
30 Vitamin A deficiency* (% pregnant women)
Childhood obesity† (%) weight less than 2·5 kg, and high birthweight is
25
classed as a birthweight heavier than 4·0 or 4·5 kg.16
Prevalence (%)
20 Other terms used to indicate neonatal size include
15 intrauterine growth restriction (IUGR), small for
gestational age, and large for gestational age. For
10
simplicity, we define low birthweight as all babies with
5 a birthweight less than 2·5 kg, including those who
0 are small for gestational age, and high birthweight as
World Africa Asia Europe Latin America North Oceania children with a birthweight greater than 4·0–4·5 kg,
and Caribbean America
Region
including those who are large for gestational age. A
premature infant—ie, who is born before 37 weeks of
Figure 2: Worldwide prevalence of factors affecting programming of renal disease, by UN region gestation—is generally low birthweight, which might
Error bars represent the range of prevalence, by region. *Excludes countries with 2005 gross domestic product of
be an appropriate weight for gestational age if growth
US$15 000 or higher, where vitamin A deficiency is presumed absent. †Obesity figures for North America and
Europe are extrapolated from data for “developed countries”. Childhood obesity is defined as two or more SD from occurred normally until birth, but could be small for
weight-for-height median. Data are pooled from references 11–15. gestational age if growth were restricted.16
274 www.thelancet.com Vol 382 July 20, 2013
Series
Nephron number in children and adults
Effect on kidney
The total number of nephrons in the normal adult
human kidney varies widely.17 The average number of Prenatal
nephrons per kidney for many years was assumed to be Maternal vitamin A deficiency Small infant kidney size
between 900 000 and 1 000 000, yet the observed range Low birthweight Decreased nephron number
between individuals varies more than tenfold.17,18 In the Growth restriction Reduced glomerular filtration rate at 7·5 years
largest study to date,18 total nephron number ranged Prematurity Decreased nephron number; reduced kidney
size in growth-restricted children
from 210 332 to 2 702 079 in 176 adults of African-
Genetics
American ethnic origin and from 227 327 to 1 660 232 in
RET (1476A) polymorphism 10% reduction in newborn kidney volume
132 white individuals.
Birthweight correlates linearly with nephron number PAX2 AAA haplotype 10% reduction in newborn kidney volume
in adults and children, and nephron number increases Combined RET (1476A) polymorphism and PAX2 23% reduction in newborn kidney volume
AAA haplotype
by 257 426 per kg increase in birthweight,19 suggesting
I/D ACE polymorphism 8% reduction in newborn kidney volume
(by extrapolation) that nephron numbers are lower
BMPR1A rs7922846 polymorphism 13% reduction in newborn kidney volume
in people with a low birthweight. Although nephron
OSR1 rs12329305(T) polymorphism 12% reduction in newborn kidney volume
numbers have been measured rarely in adults of
Combined OSR1 and RET polymorphisms 22% reduction in newborn kidney volume
known low birthweight, nephron numbers are reduced
Combined OSR1 and PAX2 polymorphisms 27% reduction in newborn kidney volume
significantly in infants with low birthweight.20,21 The total
number of nephrons in an adult human kidney reflects ALDH1A2 rs7169289(G) polymorphism 22% increase in newborn kidney size
the number of nephrons formed during development Postnatal
(nephron endowment) minus the number of nephrons Renal failure Decreased nephron number
subsequently lost; therefore, cumulative injury over time Growth restriction Reduced glomerular filtration rate at 7·5 years;
increased odds of renal dysfunction at 6·4 years
could contribute to a kidney reaching a very low nephron
Catch-up growth, childhood and adolescent overweight Increased glomerular volume; faster
number, leading to disease.17 Human nephrogenesis or obesity progression of renal disease
ends at around 36 weeks of gestation, after which
no new nephrons can form.20 In an Australian study, Adapted from reference 23, with permission of Lippincott Williams and Wilkins. RET=tyrosine kinase receptor.
PAX2=paired box gene 2. ACE=angiotensin-converting enzyme. OSR=Odd-Skipped related. BMPR=bone morphogenic
nephron number in 15 infants who died before the age protein receptor. ALDH=aldehyde dehydrogenase. See appendix for relevant references.
of 3 months ranged 4·5-fold, from 246 181 to 1 106 062,
suggesting that much of the variation in nephron Table 1: Developmental factors associated with nephron number, kidney size, and function
number in adults is established before birth.22
Developmental determinants of low these are major risk factors for low birthweight.27 As See Online for appendix
nephron number outlined in a Comment linked to this Series,28 risk of pre-
The most robust clinical surrogates for low nephron eclampsia in a mother is increased if she herself was low
number are low birthweight and prematurity. However, birthweight (odds ratio 1·69, 95% CI 1·4–2·02),29 pre-
not all factors that affect nephron number result in low mature (1·95, 1·54–2·47),30 or either of the mother’s
birthweight, therefore, awareness of risk factors for low parents were born after pre-eclamptic pregnancies (2·2,
nephron number per se is also important (table 1). The 2·0–2·4),31 showing the complexity of intergenerational
most important risk factors, some of which could be programming. Prepregnancy maternal chronic kidney
modifiable with public health interventions, include disease and hypertension are also relevant risk factors for
maternal health and nutrition, prenatal and postnatal pre-eclampsia, low birthweight, and preterm delivery.32 In
environments, prematurity, and genetic predisposition.24 a Cuban cohort,21 maternal hypertension was associated
with low birthweight, which in turn was associated with
Maternal factors low nephron number.
Figure 1 shows maternal health factors that could affect
the risk of low birthweight and prematurity and should Gestational diabetes
be judged risk factors for low nephron number in The worldwide prevalence of gestational diabetes is
offspring.25 Mothers who themselves were low birth- poorly recorded but is reported as 0·1–25·3%.33 Maternal
weight, compared with mothers who were not, were obesity, now present in 15–20% of pregnancies, is a
more likely to have babies of low birthweight (odds strong risk factor for gestational diabetes, as is maternal
ratio 1·8, 95% CI 1·3–2·5), a finding that is independent prematurity.30,34 In experimental models, maternal hyper-
of socioeconomic factors, suggesting a genetic or epi- glycaemia is associated with reduced nephron number,
genetic intergenerational effect.26 raised blood pressure, microalbuminuria, and dimin-
ished glomerular filtration rate in offspring.35 In adult
Hypertensive disorders during pregnancy children whose mother had diabetes, compared with
Disorders linked to high blood pressure in pregnancy those who had a diabetic father, renal functional reserve
were noted in 8·4 million women worldwide in 2004, and was decreased, suggesting a reduction in nephron
www.thelancet.com Vol 382 July 20, 2013 275
Series
number was acquired during exposure to gestational prematurity is a risk factor for acute kidney injury, which
diabetes.36 Maternal diabetes is also associated with a is an independent predictor of mortality and subsequent
threefold increased risk of renal agenesis and dysgenesis; chronic kidney disease in very low birthweight infants.16
therefore, hyperglycaemia strongly affects fetal renal
development.37 Furthermore, gestational diabetes is Postnatal factors
sometimes associated with high birthweight in infants, Human nephrogenesis is complete at term; however,
which is a known risk factor for subsequent hypertension, ongoing nephrogenesis has been recorded up to 40 days
type 2 diabetes, renal disease, and cardiovascular disease, after birth in infants born before 30 weeks of gestation.44
although the effect on nephron number is unknown.38 Thus, a window of vulnerability exists in preterm infants,
during which time kidney development can be affected
Maternal behaviour (table 1). Indeed, extrauterine growth restriction was
Smoking by the mother has been associated with low associated with a significantly lower glomerular filtration
birthweight and low nephron number.21 Alcohol rate in very low birthweight children at a mean age of
consumption is linked to a dose-dependent increased 7·6 years; conversely, the frequency of renal impairment
risk of prematurity and fetal growth restriction.39 In was 33% lower at 6·4 years among very low birthweight
experimental models, gestational alcohol exposure children who had gained more weight in neonatal
impaired embryonic ureteric bud branching resulting intensive care, showing the importance of early nutrition
in low nephron number, and it could be a risk in on kidney development.45,46 Nephron number was low in
human beings.40 premature infants who developed renal failure before
death, although whether renal failure was a cause or
Prenatal factors outcome of low nephron number is unknown.44
Maternal diets deficient in protein, total calories, or iron Many premature infants receive perinatal drugs
all reduce nephron numbers in experimental models and such as non-steroidal anti-inflammatory agents, gluco-
are often associated with low birthweight.9 In human corticoids, and aminoglycosides. Extrapolating from
beings, maternal protein and micronutrient deficiencies experimental models, these and other drugs can affect
are common in developing countries, and maternal nephron number and increase the risk of acute kidney
malnutrition, underweight, iron deficiency, and anaemia injury in infants.47 However, follow-up of people whose
are all recognised risk factors for low birthweight.41 mother was exposed to betamethasone for 48 h before
Vitamin A deficiency is also highly prevalent among birth did not show any increase in blood pressure
pregnant women worldwide (figure 2).12 In animals, at age 30 years compared with those whose mother
maternal diets deficient in vitamin A, resulting in had received a placebo.48 Short-term steroids might,
amounts similar to those seen in deficient people, induce therefore, not affect kidney development, but the effects
a dose-dependent reduction in nephron number, whereas of such common drugs on human nephrogenesis merit
vitamin A supplementation augments nephron number.42 further study.
Importantly, vitamin A deficiency alone does not cause
low birthweight, suggesting the effect of deficiency could Genetics
be overlooked if normal birthweight was presumed to Rare genetic and congenital abnormalities resulting in
exclude an adverse developmental environment. The renal hypoplasia contribute to about half of all cases of
active metabolite of vitamin A—retinoic acid—regulates childhood end-stage renal disease.49 Common poly-
transcription of RET, a tyrosine kinase receptor important morphisms in several genes known to participate in
for kidney development. Plausibly, therefore, vitamin A kidney development correlate with altered gene tran-
intake could be a vital determinant of nephron number. scription and newborn kidney size, which is proportional
Indeed, vitamin A deficiency in Indian mothers was to nephron number (table 1).22 These studies have been
associated with significantly lower newborn adjusted undertaken mainly in white populations, therefore
renal volume in their offspring compared with babies implications for other populations need to be investi-
born to Canadian mothers who were vitamin A replete, gated.22,50,51 The molecular mechanisms regulating
probably reflecting lower nephron numbers (table 1).43 nephrogenesis are reviewed elsewhere.52 Individual
permutations of these genetic variants could account for
Prematurity the wide variability seen in human nephron numbers,
About 9·6% of liveborn babies are premature (figure 2), because some mutations reduce and some augment
and prematurity is associated with raised blood pressure, kidney volume. Interactions between genetic poly-
renal disease, and cardiovascular disease in later life.13,16 morphisms and environmental circumstances during
Nephron number correlates with gestational age; in kidney development have not been studied. Gene micro-
premature infants, nephrogenesis might continue for a array analysis of neonatal kidneys has shown global
period after birth, although glomeruli are large and downregulation of gene expression in experimental
abnormal and renal maturation seems accelerated.20,44 models of maternal low protein diet or placental insuffi-
Consistent with these morphological abnormalities, ciency.53 Therefore, altered levels of gene expression
276 www.thelancet.com Vol 382 July 20, 2013
Series
resulting from a polymorphism could become even
Association with nephron number
more amplified under conditions of superimposed
maternal nutrient deficiency, further decreasing Clinical surrogate
nephron number.54 Low birthweight Increase of 257 426 glomeruli per kidney, per kg increase in birthweight
Prematurity Decrease in glomerular number, proportional to gestational age, in premature
compared with term infants
Clinical surrogates for nephron number
Sex Nephron number 12% lower in women
At present, all reports of human nephron number have
Age 3676 fewer glomeruli per kidney per year of age older than 18 years (nephron loss)
come from kidneys obtained at autopsy. In view of the
Adult height 28 000 more glomeruli per cm increase in height
current reliance on autopsy specimens, surrogate
Kidney mass 23 459 more glomeruli per g of kidney tissue (in infants)
markers for nephron number are important (table 2).
Glomerular volume Inverse correlation between glomerular volume and nephron number
Similar to total nephron number, mean glomerular
volume varied up to tenfold in an Australian series of Ethnic origin Reduced number in Indigenous Australians compared with white and black
US population
420 kidneys from people in five ethnic groups.55 Total
Possible correlates
nephron number varies inversely with mean glomerular
Gestational diabetes Decrease in renal functional reserve in offspring of diabetic mothers versus
volume.17 An increase in glomerular volume probably exposure diabetic fathers
reflects compensatory hypertrophy and hyperfiltration
in individual nephrons. Indeed, total filtration surface Adapted from reference 23, with permission of Lippincott Williams and Wilkins. See appendix for relevant references.
area is fairly well preserved in kidneys with low nephron Table 2: Clinical surrogates for nephron number
number, possibly at the expense of increased glomerular
pressure, which can accelerate further nephron loss.18
Moreover, increased glomerular size is a predictor of Panel: Clinical associations with programming of kidney function
poorer renal outcomes in African-American populations,
Native Americans (Pima Indians), and Indigenous Low birthweight or prematurity
Australians, and without other causes should be judged • Increased blood pressure
a surrogate for low nephron number.9 In view of the • Salt sensitivity
heterogeneity and hypertrophy occurring in glomeruli, • Proteinuria
kidney size does not correlate consistently with nephron • Reduced glomerular filtration rate
number in adults, but the relation seems linear in • Reduced renal functional reserve
infants younger than 3 months.22,56 • Accelerated progression of primary renal disease
• Chronic kidney disease
Birthweight, prematurity, and blood pressure • End-stage kidney disease
Studies of monozygotic twins, in which the twin who • Death
weighed the least subsequently had higher blood pres- Low nephron number
sure, suggest that environmental programming could be • Increased blood pressure
more crucial than genetic factors.57 Low birthweight and • Increased glomerular volume
prematurity have been associated consistently with • Possible predisposition to renal failure in neonates
increased risk of higher blood pressure in later life
(panel).58,59 In a meta-analysis of 27 studies,59 a 2·28 mm Hg Reduced renal size
(95% CI 1·24–3·33) increase in systolic blood pressure • Increased blood pressure
was recorded in individuals whose birthweight was less • Salt sensitivity
than 2·5 kg, compared with those heavier than 2·5 kg. • Reduced glomerular filtration rate
Unfortunately, in most studies to date, a distinction has High birthweight or maternal diabetes
not been made between low birthweight as a result of • Proteinuria
growth restriction at any gestational age and prematurity • Reduced renal functional reserve
with appropriate size for gestational age. Therefore, the • End-stage kidney disease
potential for unmeasured confounding or effect modifi-
cation by gestational age, growth restriction, or both must Rapid increase in weight or body-mass index in childhood and adolescence,
be borne in mind.16 particularly after low birthweight
Findings of a systematic review of ten studies showed • Faster progression of renal disease
that, in preterm babies born at a mean gestational age of • Larger glomeruli
30·2 weeks and with a mean birthweight of 1·28 kg, blood • Higher blood pressure
pressures in later life were 2·5 mm Hg higher (95% CI • Diabetes and impaired glucose tolerance
1·7–3·3) than in infants born at term.58 Prematurity has • Cardiovascular disease and death
been associated predominantly with high, but still normal, • Obesity
blood pressures, because cohorts studied are still fairly • Metabolic syndrome
young. Overt hypertension has been recorded in two Adapted from reference 23, with permission of Lippincott Williams and Wilkins.
studies of premature babies: in the first, the children
www.thelancet.com Vol 382 July 20, 2013 277
Series
were aged 2 years and the prevalence of hypertension associated with a salt-induced increase in blood pressure
(defined as systolic or diastolic blood pressure greater (panel).66,67 Salt sensitivity in young adults and children
than the 95th percentile) was 30% overall; and in the correlates inversely with birthweight, independent of
second, pregnant women were aged 25 years and chronic glomerular filtration rate, suggesting a primary defect in
hypertension was present in 1·4% of those born preterm renal sodium handling.68,69
compared with 0·8% born at term (odds ratio 1·7, 95% CI Evidence that hypertension is not eliminated despite
1·32–2·20).30,60 Researchers have tried to dissect the relative normalisation of nephron number in some experimental
roles of prematurity and growth restriction; some suggest models suggests that additional factors participate in
prematurity alone is the predominant risk factor, whereas developmental programming of hypertension.70 Experi-
others judge small for gestational age to be most important mental work has shown alterations in expression of renal
when ascertaining risk of raised blood pressure and kidney tubule sodium transporters and systemic changes in
disease.61 These differences show the complex interplay of vascular function, neuroendocrine adaptations to stress,
intrauterine and extrauterine events and timing of insults, insulin sensitivity, and sympathetic nervous system
which vary considerably in premature infants.16 activity.70 Nephron number, therefore, is not the sole pro-
Because odds ratios for risk of high blood pressure grammed risk factor for hypertension, but it is likely to
were similar between the meta-analysis of low birth- exacerbate any risk and contribute to kidney disease.
weight59 and the systematic review of prematurity,58 and
since prematurity does not account for all babies with Renal function, birthweight, and nephron mass
low birthweight, both low birthweight and prematurity Glomerular filtration rate
must be deemed important risk factors for high blood Without compensatory hyperfiltration, a kidney with a
pressure. The association starts in early childhood reduced number of nephrons should have a diminished
and becomes augmented in adulthood, at which stage glomerular filtration rate. Indeed, glomerular filtration
blood pressures typically reach hypertensive ranges, rate extrapolated from amikacin clearance on day 1 of life,
suggesting the programming effects are compounded by preceding any compensatory adaptation, was decreased
growth, age, and lifestyle. significantly in premature and low-birthweight infants
compared with term controls.71 Glomerular filtration rate
Nephron number and blood pressure measured by inulin clearance was significantly lower at
In rodents, nephrogenesis continues for up to 7–10 days age 7·6 years in children who had been born premature
after birth, providing a window—similar to that seen in and had severe growth restriction compared with non-
premature infants—during which postnatal events can growth-restricted controls.45 In this study, the effects were
affect nephron number. In rats of low birthweight, rescue similar in children who were growth-restricted prenatally
of nephron number by optimisation of postnatal or postnatally in intensive care, again showing the
nutrition abrogated development of subsequent hyper- importance of early nutrition. In a meta-analysis of eight
tension; conversely, undernutrition after birth of rat pups studies,10 the odds ratio for reduced glomerular filtration
of normal birthweight led to lower nephron numbers rate with low birthweight was 1·79 (95% CI 1·31–2·45).
and higher blood pressure.62,63 These data accord with a
role of nephron number in hypertension. Proteinuria
In a German cohort of adults who died in accidents, Microalbuminuria is one of the earliest signs of
nephron numbers were significantly lower among glomerular hyperfiltration, and transition to macro-
those with hypertension compared with normotensive albuminuria accords with ongoing renal injury. Hoy and
controls.64 Low nephron numbers have also been colleagues72 reported an odds ratio of 2·8 (95% CI
associated with raised blood pressure in Indigenous 1·26–6·31) for macroalbuminuria in Indigenous
Australians and white populations from the USA and Australians who were low birthweight compared with
Australia, although birthweights were unknown.65 those of normal birthweight. In this population,
The relation between nephron number and blood albuminuria was associated with a significant increase
pressure in people of African origin seems less clear, in cardiovascular and renal deaths, emphasising its
but glomerular volume is a significant independent clinical relevance.73 Many study findings have since
predictor of high blood pressure in this population.65 confirmed this association, reflected in an odds ratio of
Additional factors probably contribute to hypertension 1·81 (95% CI 1·19–2·77) for albuminuria in low-birth-
in African populations, but the effect of birthweight weight individuals in a meta analysis of nine studies.10 In
or the contribution of nephron number to severity of Native Americans (Pima Indians), however, the relation
hypertension, for example, cannot be excluded. is U-shaped, with an increased risk of proteinuria within
Observations of normalisation of glomerular filtra- those whose birthweight was less than 2·5 kg and
tion surface area despite low nephron numbers65 greater than 4·5 kg.38 This population has a high
argue against the hypothesis that sodium excretion is prevalence of gestational diabetes, exposure to which
restricted. However, in patients and in experimental was the strongest predictor of proteinuria in the high-
models, low birthweight and low nephron number were birthweight people.38
278 www.thelancet.com Vol 382 July 20, 2013
Series
Chronic kidney disease and end-stage renal disease in body-mass index (BMI), even in children with a
Amplified progression of primary renal diseases has normal birthweight, has been associated consistently
been noted in people with low birthweight.9 In individuals with amplified risk of adult hypertension, type 2
with diabetes, prevalence of nephropathy has been diabetes, and cardiovascular disease.80–82 This effect
associated with both low and high birthweight, and short grows as the child ages: upward crossing of weight
stature.38,74 All these observations suggest that abnormal or BMI percentiles in mid-childhood or adolescence
fetal growth increases long-term risk of renal disease is associated with strong adverse effects on later risk,
(panel). Consistent with this hypothesis, the odds ratio whereas upward crossing in infancy (younger than
for chronic kidney disease (including end-stage renal 1 year) has no or little effect on later blood pressure and
disease) associated with low birthweight was 1·73 could protect against diabetes.82–84 The gain in childhood
(95% CI 1·44–2·08) from 18 studies.10 In population- BMI associated with increased risk of adult disease is not
based studies, a U-shaped relation has been reported always excessive in terms of absolute number. In many
between birthweight and risk of chronic kidney disease developing countries, children who grow rapidly can still
or end-stage renal disease, suggesting high birthweight be small by international weight standards, but upwards
is also important.8,75,76 In some studies, the programmed crossing of BMI percentiles seems to be the important
risk of chronic kidney disease seems to be greater in factor.82–84 Therefore, one cross-sectional measurement
men.75 However, the differential effect of sex on renal of a child’s weight or BMI could be misleading in
programming is quite variable and needs further study.76,77 such circumstances, emphasising the need for growth
Similar to programming of blood pressure risk, tracking in early childhood.
nephron number is unlikely to be the sole factor con- Children born with a low birthweight who have an
tributing to risk of renal disease, and a low nephron adequate nutrient supply tend to gain weight rapidly.5
number will probably not be sufficient to cause Among 22-year-old British adults, systolic blood pressure
renal disease without additional variables. Moreover, increased by 1·3 mm Hg (95% CI 0·3–2·3) for every
developmental programming of related disorders—eg, SD decrease in birthweight and rose by 1·6 mm Hg
type 2 diabetes, cardiovascular disease, insulin resis- (0·6–2·7) for every SD gain in childhood weight between
tance and obesity—could increase renal risk further.4,16 age 1 year and 10 years.85 An association is well
Programming of these disorders might take place recognised between rapid childhood weight gain and
simultaneously in a developing fetus, depending on raised blood pressure and increased arterial stiffness,
timing and nature of the insults. All infants subjected to which is sometimes already evident in childhood.83
adverse intrauterine conditions should be judged at risk In a study of 216 771 Scandinavian adults,81 those
for all these disorders. with a birthweight of 2·5 kg and a BMI of 17·7 kg/m²
(overweight) at age 7 years had a 44% increased risk of
Effect of childhood weight gain on kidney cardiovascular disease in adulthood compared with those
disease and function with a median birthweight of 3·4 kg and a BMI of
Postnatal malnutrition and clinical circumstances can 15·3 kg/m² (normal) at age 7 years. The highest risk
affect nephrogenesis, childhood renal function, and long- of raised blood pressure and cardiovascular disease,
term risk of renal disease (figure 1).16,44,46 In rats with low therefore, was present in children born with low
birthweight, low nephron numbers were restored to birthweight who became heavy.81 Importantly, BMI was
normal and development of hypertension was abrogated associated strongly and positively with cardiovascular
by provision of adequate postnatal nutrition.62 When low- disease risk in this study, independent of birthweight,
birthweight rats were overfed after birth, nephron showing the importance of childhood obesity itself as a
numbers remained low and the rodents developed obesity, risk factor for adult disease.
hypertension, and renal injury over time.78 In rats with The prevalence of childhood obesity is increasing
normal birthweight that were overfed postnatally, despite worldwide (figure 2).14 Risk factors for obesity include
a higher-than-normal nephron number, blood pressure, high birthweight, exposure to gestational diabetes, and
proteinuria, and glomerulosclerosis were all increased in early postnatal weight gain.86 These factors are also
adulthood.79 Taken together, these data suggest that associated independently with altered nephrogenesis
normalisation of postnatal nutrition can be beneficial, but and increased risk of hypertension, type 2 diabetes,
overfeeding is probably deleterious. In infants, postnatal and renal dysfunction (figure 1). Furthermore, obesity
weight gain and nutrition have been implicated in per se is a risk factor for progression of renal disease;
developmental programming of adult disease.80 therefore, superimposition of the burden of obesity on a
Catch-up growth in children who were of low small kidney with fewer nephrons is likely to compound
birthweight has long been advocated, particularly the risk and act as a second hit, accelerating renal
in developing countries, to boost resilience against disease progression.87 How can we optimise postnatal
infections and reduce risk of undernutrition, stunting, growth and positively change any subsequent disease
and cognitive impairment.5 However, in many popu- risk, particularly in low-birthweight infants? Avoidance
lations worldwide, accelerated weight gain or an increase of obesity seems to be a safe guiding principle.
www.thelancet.com Vol 382 July 20, 2013 279
Series
Early growth and kidney function convincing.3 Low birthweight and prematurity are
The association of rapid childhood weight gain with high associated with raised blood pressure and decreased
blood pressure, diabetes, and obesity is likely to com- renal function, manifesting in early childhood, which
pound any primary programmed renal risk. Indeed, in a could progress to overt disease in adulthood. Nutrition is
retrospective analysis of 80 children with proteinuric a cornerstone of this association. Maternal nutrition and
kidney disease, renal disease progressed fastest in those health before and during pregnancy are crucial for fetal
who had been premature and became obese.88 Glomerular growth and for development of a kidney with enough
size was increased in all obese children, whether premature nephrons to maintain homoeostasis in response to
or term, whereas kidney size remained small in all those dietary and metabolic stresses and to sustain function in
who had been premature, independent of obesity. the face of superimposed nephron loss. Early postnatal
Similarly, among infants with very low birthweight who nutrition, particularly after premature birth, is also
developed neonatal acute kidney injury, excessive weight important for renal development. Furthermore, upward
gain was a predictor of poorer renal function at a mean age crossing of weight or BMI percentiles after the infant
of 7·5 (SD 4·6) years.89 The effect of infant weight gain on period programmes risk of hypertension and renal
long-term renal function remains unknown. disease. Developmental programming has an inter-
Several mechanisms have been proposed to explain the generational effect, because low birthweight increases
amplification of renal and cardiovascular disease risk by the risk of maternal pre-eclampsia, obesity, and
rapid weight gain after growth restriction. One possibility gestational diabetes, which all in turn further compound
is development of premature senescence.90 Cellular future risks in their offspring.
senescence is a state of growth arrest induced by up- Growing knowledge and understanding of the patho-
regulation of the cell-cycle inhibitors P53, P21, and physiology of developmental programming has identified
P16INK4a.91 Upregulation of the genes for these proteins at-risk populations that could be targets for screening
can be induced by progressive telomere shortening, and interventions to interrupt this cycle. Identification
which takes place with cell replication and is a robust of nutritional deficiencies within populations (eg,
marker of ageing, and by reactive oxygen species induced vitamin A deficiency) ought to prompt public health
by cellular stress.91 interventions to correct these deficiencies well before
Chronic cardiovascular and renal diseases are pregnancy. Adequate antenatal care should identify
associated with increased expression of senescence women who develop pre-eclampsia and gestational
markers.92 In experimental models, low birthweight diabetes, optimise their care during pregnancy, and
followed by rapid postnatal weight gain was associated lead to lifestyle education and lifelong screening of
with shorter telomeres and amplified expression of these women for later disease. Currently, few women
senescence markers in the kidneys, heart, and aorta, worldwide are screened for gestational diabetes.33
in addition to premature death, which all accord with With the rising prevalence of obesity, such screening
accelerated ageing.90,93,94 Low birthweight was also asso- should be more widespread. Similarly, according to
For more on Childinfo see ciated with higher renal and cardiovascular mortality the UNICEF project Childinfo, only about 60% of all
http://www.childinfo.org in an Indigenous Australian cohort, which accords with children are weighed at birth, and this number should
these experimental findings.95 increase. Several interventions to reduce maternal and
Premature senescence in the kidney might be childhood malnutrition, where prevalent, have been
attributable to ongoing hyperfiltration injury in kidneys effective: supplementation of protein energy intake
with a few nephrons, compounded by a rapid increase during pregnancy has reduced the risk of babies born
in body size. In human beings, leucocyte telomere at term with low birthweight by 32%; supplementation
length did not differ between British babies of low and of multiple micronutrients in pregnancy decreased the
normal birthweight,96 but among 5-year-old children proportion of children with low birthweight by 16%;
from Bangladesh, telomeres were significantly shorter and implementation of malaria prophylaxis diminished
in those who were of low birthweight.97 the risk of low-birthweight babies by 37%.99 Vitamin A
Senescence is linked to oxidative stress. In children supplementation did not affect birthweight but did lower
born small for gestational age, compared with controls of neonatal mortality (relative risk 0·8, 95% CI 0·66–0·96),
appropriate size for gestational age, markers of oxidative whereas the relative risk of pre-eclampsia was 0·45
stress were highest in those who experienced catch-up (0·31–0·65) with maternal calcium supplementation.99,100
growth.98 The link between nephron numbers, catch-up As far as we know, no study has followed up the offspring
growth, premature senescence, and development of of such supplemented pregnancies to ascertain whether
hypertension and renal disease seems plausible, but it risk of adult disease has been altered, but we should
has not yet been confirmed. assume a positive effect.
In later life, regular cardiovascular exercise abrogates
Conclusion the metabolic outcomes of being small at birth among
The association between fetal and childhood development men.101 Identification of at-risk pregnancies and offspring
and increased risk of adult disease is now quite of both high and low birthweight should prompt
280 www.thelancet.com Vol 382 July 20, 2013
Series
maternal education to optimise childhood nutrition 17 Puelles VG, Hoy WE, Hughson MD, Diouf B, Douglas-Denton RN,
and activity to prevent obesity. Prematurity and low Bertram JF. Glomerular number and size variability and risk for
kidney disease. Curr Opin Nephrol Hypertens 2011; 20: 7–15.
birthweight are among the top ten contributors to the 18 Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE.
global burden of disease, calculations that might not Human nephron number: implications for health and disease.
always have included the long-term costs of programmed Pediatr Nephrol 2011; 26: 1529–33.
19 Hughson M, Farris AB, Douglas-Denton R, Hoy WE, Bertram JF.
adult non-communicable diseases.1,27 Acknowledgment of Glomerular number and size in autopsy kidneys: the relationship to
the role of developmental programming in hypertension birth weight. Kidney Int 2003; 63: 2113–22.
and renal disease risk, and implementation of locally 20 Hinchliffe SA, Sargent PH, Howard CV, Chan YF, van Velzen D.
Human intrauterine renal growth expressed in absolute number of
adapted pre-emptive strategies in individual countries, glomeruli assessed by the disector method and Cavalieri principle.
will have important long-term benefits in terms of future Lab Invest 1991; 64: 777–84.
health, productivity, and cost savings worldwide. 21 Manalich R, Reyes L, Herrera M, Melendi C, Fundora I.
Relationship between weight at birth and the number and size
Contributors of renal glomeruli in humans: a histomorphometric study.
VAL developed the idea and outline for the paper. All authors Kidney Int 2000; 58: 770–73.
contributed to writing. JFB contributed to the section on nephron 22 Zhang Z, Quinlan J, Hoy W, et al. A common RET variant is
number in children and adults. VAL and BMB contributed to the section associated with reduced newborn kidney size and function.
on developmental determinants of nephron number. WEH, VAL, and J Am Soc Nephrol 2008; 19: 2027–34.
BEV contributed to sections on clinical outcomes associated with 23 Luyckx VA, Mueller TF. Clinical importance of nephron mass.
birthweight and nephron number. CF and SEO contributed to the In: Schrier RW, Neilson E, Molitoris B, Coffman T, Falk R, eds.
section on the effect of childhood weight gain on kidney function. Schrier’s diseases of the kidney and urinary tract, 9th edn.
Philadelphia: Lippincott Williams and Wilkins, 2012: pp 46–73.
Conflicts of interest
24 Hoy WE, Ingelfinger JR, Hallan S, Hughson MD, Mott SA,
We declare that we have no conflicts of interest.
Bertram JF. The early development of the kidney and implications
References for future health. J Dev Origins Health Dis 2010; 1: 216–33.
1 Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment 25 Valero De Bernabe J, Soriano T, Albaladejo R, et al. Risk factors for
of burden of disease and injury attributable to 67 risk factors and low birth weight: a review. Eur J Obstet Gynecol Reprod Biol 2004;
risk factor clusters in 21 regions, 1990–2010: a systematic analysis 116: 3–15.
for the Global Burden of Disease Study 2010. Lancet 2012; 26 Collins JW, Rankin KM, David RJ. Low birth weight across
380: 2224–60. generations: the effect of economic environment.
2 Lozano R, Naghavi M, Foreman K, et al. Global and regional Matern Child Health J 2010; 15: 438–45.
mortality from 235 causes of death for 20 age groups in 1990 and 27 WHO. Global burden of disease: 2004 update. Geneva: World
2010: a systemayic analysis for the Global Burden of Disease Study Health Organization, 2008.
2010. Lancet 2012; 380: 2095–128. 28 Vikse BE. Pre-eclampsia and the risk of kidney disease. Lancet 2013;
3 Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull published online May 31. http://dx.doi.org/10.1016/S0140-
2001; 60: 5–20. 6736(13)60741-2.
4 Hanson M, Gluckman P. Developmental origins of 29 Zetterstrom K, Lindeberg S, Haglund B, Magnuson A, Hanson U.
noncommunicable disease: population and public health Being born small for gestational age increases the risk of severe
implications. Am J Clin Nutr 2011; 94 (6 suppl): 1754S–8S. pre-eclampsia. BJOG 2007; 114: 319–24.
5 Jain V, Singhal A. Catch up growth in low birth weight infants: 30 Boivin A, Luo ZC, Audibert F, et al. Pregnancy complications
striking a healthy balance. Rev Endocr Metab Disord 2012; 13: 141–47. among women born preterm. CMAJ 2012; 184: 1777–84.
6 Fall C. Maternal nutrition: effects on health in the next generation. 31 Skjaerven R, Vatten LJ, Wilcox AJ, Ronning T, Irgens LM, Lie RT.
Indian J Med Res 2009; 130: 593–99. Recurrence of pre-eclampsia across generations: exploring fetal and
7 Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure: maternal genetic components in a population based cohort.
less of one, more the other? Am J Hypertens 1988; 1: 335–47. BMJ 2005; 331: 877.
8 Lackland DT, Bendall HE, Osmond C, Egan BM, Barker DJ. Low 32 Nevis IF, Reitsma A, Dominic A, et al. Pregnancy outcomes in
birth weights contribute to high rates of early-onset chronic renal women with chronic kidney disease: a systematic review.
failure in the Southeastern United States. Arch Intern Med 2000; Clin J Am Soc Nephrol 2011; 6: 2587–98.
160: 1472–76. 33 Jiwani A, Marseille E, Lohse N, Damm P, Hod M, Kahn JG.
9 Luyckx VA, Brenner BM. The clinical importance of nephron mass. Gestational diabetes mellitus: results from a survey of country
J Am Soc Nephrol 2010; 21: 898–910. prevalence and practices. J Matern Fetal Neonatal Med 2011;
10 White SL, Perkovic V, Cass A, et al. Is low birth weight an 25: 600–10.
antecedent of CKD in later life? A systematic review of 34 Norman JE, Reynolds RM. The consequences of obesity and excess
observational studies. Am J Kidney Dis 2009; 54: 248–61. weight gain in pregnancy. Proc Nutr Soc 2011; 70: 450–56.
11 WHO. World health statistics 2012. 2012. http://apps.who.int/iris/ 35 Amri K, Freund N, Vilar J, Merlet-Bénichou C, Lelièvre-Pégorier M.
bitstream/10665/44844/1/9789241564441_eng.pdf (accessed Adverse effects of hyperglycemia on kidney development in rats:
Feb 20, 2013). in vivo and in vitro studies. Diabetes 1999; 48: 2240–45.
12 WHO. Global prevalence of vitamin A deficiency in populations at 36 Abi Khalil C, Travert F, Fetita S, et al. Fetal exposure to maternal
risk 1995–2005: WHO global database on vitamin A deficiency. type 1 diabetes is associated with renal dysfunction at adult age.
Geneva: World Health Organization, 2009. Diabetes 2010; 59: 2631–36.
13 Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm 37 Davis EM, Peck JD, Thompson D, Wild RA, Langlois P.
birth: a systematic review of maternal mortality and morbidity. Maternal diabetes and renal agenesis/dysgenesis.
Bull World Health Organ 2010; 88: 31–38. Birth Defects Res A Clin Mol Teratol 2010; 88: 722–27.
14 de Onis M, Blossner M, Borghi E. Global prevalence and trends of 38 Nelson RG, Morgenstern H, Bennett PH. Birth weight and renal
overweight and obesity among preschool children. Am J Clin Nutr disease in Pima Indians with type 2 diabetes mellitus.
2010; 92: 1257–64. Am J Epidemiol 1998; 148: 650–56.
15 UNICEF, WHO. Low birthwieght: country, regional and global 39 Patra J, Bakker R, Irving H, Jaddoe VW, Malini S, Rehm J.
estimates. Geneva: United Nations Children’s Fund and World Dose-response relationship between alcohol consumption before
Health Organization, 2004. and during pregnancy and the risks of low birthweight, preterm
16 Abitbol CL, Rodriguez MM. The long-term renal and birth and small for gestational age (SGA)—a systematic review and
cardiovascular consequences of prematurity. Nat Rev Nephrol meta-analyses. BJOG 2011; 118: 1411–21.
2012; 8: 265–74.
www.thelancet.com Vol 382 July 20, 2013 281
Series
40 Gray SP, Kenna K, Bertram JF, et al. Repeated ethanol exposure 62 Wlodek ME, Mibus A, Tan A, Siebel AL, Owens JA, Moritz KM.
during late gestation decreases nephron endowment in fetal sheep. Normal lactational environment restores nephron endowment and
Am J Physiol Regul Integr Comp Physiol 2008; 295: R568–74. prevents hypertension after placental restriction in the rat.
41 Bryce J, Coitinho D, Darnton-Hill I, Pelletier D, J Am Soc Nephrol 2007; 18: 1688–96.
Pinstrup-Andersen P, for the Maternal and Child Undernutrition 63 Wlodek ME, Westcott K, Siebel AL, Owens JA, Moritz KM. Growth
Study Group. Maternal and child undernutrition: effective action at restriction before or after birth reduces nephron number and
national level. Lancet 2008; 371: 510–26. increases blood pressure in male rats. Kidney Int 2008; 74: 187–95.
42 Merlet-Benichou C, Vilar J, Lelievre-Pegorier M, Gilbert T. Role of 64 Keller G, Zimmer G, Mall G, Ritz E, Amann K. Nephron number in
retinoids in renal development: pathophysiological implication. patients with primary hypertension. N Engl J Med 2003; 348: 101–08.
Curr Opin Nephrol Hypertens 1999; 8: 39–43. 65 Hoy WE, Bertram JF, Denton RD, Zimanyi M, Samuel T,
43 Goodyer P, Kurpad A, Rekha S, et al. Effects of maternal vitamin A Hughson MD. Nephron number, glomerular volume, renal disease
status on kidney development: a pilot study. Pediatr Nephrol 2007; and hypertension. Curr Opin Nephrol Hypertens 2008; 17: 258–65.
22: 209–14. 66 Nehiri T, Duong Van Huyen JP, Viltard M, et al. Exposure to
44 Rodriguez MM, Gomez AH, Abitbol CL, Chandar JJ, Duara S, maternal diabetes induces salt-sensitive hypertension and impairs
Zilleruelo GE. Histomorphometric analysis of postnatal renal function in adult rat offspring. Diabetes 2008; 57: 2167–75.
glomerulogenesis in extremely preterm infants. Pediatr Dev Pathol 67 Salazar F, Reverte V, Saez F, Loria A, Llinas MT, Salazar FJ.
2004; 7: 17–25. Age- and sodium-sensitive hypertension and sex-dependent renal
45 Bacchetta J, Harambat J, Dubourg L, et al. Both extrauterine and changes in rats with a reduced nephron number. Hypertension 2008;
intrauterine growth restriction impair renal function in children 51: 1184–89.
born very preterm. Kidney Int 2009; 76: 445–52. 68 de Boer MP, Ijzerman RG, de Jongh RT, et al. Birth weight relates
46 Kwinta P, Klimek M, Drozdz D, et al. Assessment of long-term to salt sensitivity of blood pressure in healthy adults. Hypertension
renal complications in extremely low birth weight children. 2008; 51: 928–32.
Pediatr Nephrol 2011; 26: 1095–103. 69 Simonetti GD, Raio L, Surbek D, Nelle M, Frey FJ, Mohaupt MG.
47 Schreuder MF, Bueters RR, Huigen MC, Russel FG, Masereeuw R, Salt sensitivity of children with low birth weight. Hypertension 2008;
van den Heuvel LP. Effect of drugs on renal development. 52: 625–30.
Clin J Am Soc Nephrol 2011; 6: 212–17. 70 Baum M. Role of the kidney in the prenatal and early postnatal
48 Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors programming of hypertension. Am J Physiol Renal Physiol 2010;
after antenatal exposure to betamethasone: 30-year follow-up of a 298: F235–47.
randomised controlled trial. Lancet 2005; 365: 1856–62. 71 Schreuder MF, Wilhelm AJ, Bokenkamp A, Timmermans SM,
49 Kemper MJ, Muller-Wiefel DE. Renal function in congenital Delemarre-van de Waal HA, van Wijk JA. Impact of gestational age
anomalies of the kidney and urinary tract. Curr Opin Urol 2001; and birth weight on amikacin clearance on day 1 of life.
11: 571–75. Clin J Am Soc Nephrol 2009; 4: 1774–78.
50 Kaczmarczyk M, Goracy I, Loniewska B, et al. Association of 72 Hoy WE, Rees M, Kile E, Mathews JD, Wang Z. A new dimension
BMPR1A polymorphism, but not BMP4, with kidney size in to the Barker hypothesis: low birthweight and susceptibility to renal
full-term newborns. Pediatr Nephrol 2013; 28: 433–38. disease. Kidney Int 1999; 56: 1072–77.
51 Quinlan J, Lemire M, Hudson T, et al. A common variant of the 73 Hoy WE, Wang Z, VanBuynder P, Baker PR, McDonald SM,
PAX2 gene is associated with reduced newborn kidney size. Mathews JD. The natural history of renal disease in Australian
J Am Soc Nephrol 2007; 18: 1915–21. Aborigines, part 2: albuminuria predicts natural death and renal
52 Moritz KM, Wintour EM, Black MJ, Bertram JF, Caruana G. failure. Kidney Int 2001; 60: 249–56.
Factors influencing mammalian kidney development: 74 Rossing P, Tarnow L, Nielsen FS, Boelskifte S, Brenner BM,
implications for health in adult life. Adv Anat Embryol Cell Biol Parving HH. Short stature and diabetic nephropathy. BMJ 1995;
2008; 196: 1–78. 310: 296–97.
53 Denisenko O, Lin B, Louey S, Thornburg K, Bomsztyk K, Bagby S. 75 Li S, Chen SC, Shlipak M, et al. Low birth weight is associated with
Maternal malnutrition and placental insufficiency induce global chronic kidney disease only in men. Kidney Int 2008; 73: 637–42.
downregulation of gene expression in fetal kidneys. 76 Vikse BE, Irgens LM, Leivestad T, Hallan S, Iversen BM. Low birth
J Dev Origins Health and Dis 2011; 2: 124–33. weight increases risk for end-stage renal disease. J Am Soc Nephrol
54 Abdel-Hakeem AK, Henry TQ, Magee TR, et al. Mechanisms of 2008; 19: 151–57.
impaired nephrogenesis with fetal growth restriction: altered renal 77 Gilbert JS, Nijland MJ. Sex differences in the developmental
transcription and growth factor expression. Am J Obstet Gynecol origins of hypertension and cardiorenal disease.
2008; 199: 252.e1–7. Am J Physiol Regul Integr Comp Physiol 2008; 295: R1941–52.
55 Hoy WE, Hughson MD, Diouf B, et al. Distribution of volumes of 78 Boubred F, Daniel L, Buffat C, et al. Early postnatal overfeeding
individual glomeruli in kidneys at autopsy: association with physical induces early chronic renal dysfunction in adult male rats.
and clinical characteristics and with ethnic group. Am J Nephrol Am J Physiol Renal Physiol 2009; 297: F943–51.
2011; 33 (suppl 1): 15–20. 79 Boubred F, Buffat C, Feuerstein JM, et al. Effects of early postnatal
56 Nyengaard JR, Bendtsen TF. Glomerular number and size in hypernutrition on nephron number and long-term renal function
relation to age, kidney weight, and body surface in normal man. and structure in rats. Am J Physiol Renal Physiol 2007; 293: F1944–49.
Anat Rec 1992; 232: 194–201. 80 Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG.
57 Bergvall N, Iliadou A, Johansson S, et al. Genetic and shared Trajectories of growth among children who have coronary events as
environmental factors do not confound the association between adults. N Engl J Med 2005; 353: 1802–09.
birth weight and hypertension: a study among Swedish twins. 81 Andersen LG, Angquist L, Eriksson JG, et al. Birth weight,
Circulation 2007; 115: 2931–38. childhood body mass index and risk of coronary heart disease in
58 de Jong F, Monuteaux MC, van Elburg RM, Gillman MW, adults: combined historical cohort studies. PLoS One 2010; 5: e14126.
Belfort MB. Systematic review and meta-analysis of preterm birth 82 Fall CH, Sachdev HS, Osmond C, et al. Adult metabolic syndrome
and later systolic blood pressure. Hypertension 2012; 59: 226–34. and impaired glucose tolerance are associated with different
59 Mu M, Wang SF, Sheng J, et al. Birth weight and subsequent patterns of BMI gain during infancy: data from the New Delhi birth
blood pressure: a meta-analysis. Arch Cardiovasc Dis 2012; cohort. Diabetes Care 2008; 31: 2349–56.
105: 99–113. 83 Bansal N, Ayoola OO, Gemmell I, et al. Effects of early growth on
60 Crowther CA, Doyle LW, Haslam RR, Hiller JE, Harding JE, blood pressure of infants of British European and south Asian
Robinson JS. Outcomes at 2 years of age after repeat doses of origin at one year of age: the Manchester children’s growth and
antenatal corticosteroids. N Engl J Med 2007; 357: 1179–89. vascular health study. J Hypertens 2008; 26: 412–18.
61 Keijzer-Veen MG, Schrevel M, Finken MJ, et al. Microalbuminuria 84 Bhargava SK, Sachdev HS, Fall CH, et al. Relation of serial changes
and lower glomerular filtration rate at young adult age in subjects in childhood body-mass index to impaired glucose tolerance in
born very premature and after intrauterine growth retardation. young adulthood. N Engl J Med 2004; 350: 865–75.
J Am Soc Nephrol 2005; 16: 2762–68.
282 www.thelancet.com Vol 382 July 20, 2013
Series
85 Law CM, Shiell AW, Newsome CA, et al. Fetal, infant, and childhood 95 Hoy WE, Nicol JL. Brthweight and natural deaths in a remote
growth and adult blood pressure: a longitudinal study from birth to Australian Aboriginal community. Med J Aust 2010; 192: 14–19.
22 years of age. Circulation 2002; 105: 1088–92. 96 Akkad A, Hastings R, Konje JC, Bell SC, Thurston H, Williams B.
86 Skelton JA, Irby MB, Grzywacz JG, Miller G. Etiologies of obesity Telomere length in small-for-gestational-age babies. BJOG 2006;
in children: nature and nurture. Pediatr Clin North Am 2011; 113: 318–23.
58: 1333–54. 97 Raqib R, Alam DS, Sarker P, et al. Low birth weight is associated
87 Wang Y, Chen X, Klag MJ, Caballero B. Epidemic of childhood with altered immune function in rural Bangladeshi children: a birth
obesity: implications for kidney disease. Adv Chronic Kidney Dis cohort study. Am J Clin Nutr 2007; 85: 845–52.
2006; 13: 336–51. 98 Mohn A, Chiavaroli V, Cerruto M, et al. Increased oxidative stress
88 Abitbol CL, Chandar J, Rodriguez MM, et al. Obesity and preterm in prepubertal children born small for gestational age.
birth: additive risks in the progression of kidney disease in children. J Clin Endocrinol Metab 2007; 92: 1372–78.
Pediatr Nephrol 2009; 24: 1363–70. 99 Bhutta ZA, Ahmed T, Black RE, et al, for the Maternal and Child
89 Abitbol CL, Bauer CR, Montane B, Chandar J, Duara S, Undernutrition Study Group. What works? Interventions for
Zilleruelo G. Long-term follow-up of extremely low birth weight maternal and child undernutrition and survival. Lancet 2008;
infants with neonatal renal failure. Pediatr Nephrol 2003; 18: 887–93. 371: 417–40.
90 Ozanne SE, Hales CN. Lifespan: catch-up growth and obesity 100 Hofmeyr GJ, Lawrie TA, Atallah AN, Duley L. Calcium
in male mice. Nature 2004; 427: 411–12. supplementation during pregnancy for preventing hypertensive
91 Tarry-Adkins JL, Ozanne SE. Mechanisms of early life disorders and related problems. Cochrane Database Syst Rev 2010;
programming: current knowledge and future directions. 8: CD001059.
Am J Clin Nutr 2011; 94 (suppl): 1765S–71S. 101 Laaksonen DE, Lakka HM, Lynch J, et al. Cardiorespiratory fitness
92 Tsirpanlis G. Cellular senescence, cardiovascular risk, and CKD: and vigorous leisure-time physical activity modify the association of
a review of established and hypothetical interconnections. small size at birth with the metabolic syndrome. Diabetes Care 2003;
Am J Kidney Dis 2008; 51: 131–44. 26: 2156–64.
93 Luyckx VA, Compston CA, Simmen T, Mueller TF. Accelerated
senescence in kidneys of low-birth-weight rats after catch-up
growth. Am J Physiol Renal Physiol 2009; 297: F1697–705.
94 Tarry-Adkins JL, Martin-Gronert MS, Chen JH, Cripps RL,
Ozanne SE. Maternal diet influences DNA damage, aortic telomere
length, oxidative stress, and antioxidant defense capacity in rats.
FASEB J 2008; 22: 2037–44.
www.thelancet.com Vol 382 July 20, 2013 283
Reproduced with permission of the copyright owner. Further reproduction prohibited without
permission.
You might also like
- The Placenta and Neurodisability 2nd EditionFrom EverandThe Placenta and Neurodisability 2nd EditionIan CrockerNo ratings yet
- 08 Hypertensionaha 120 14592Document11 pages08 Hypertensionaha 120 14592DigsNo ratings yet
- Acta Paediatrica - 2019 - Svefors - Stunting Recovery From Stunting and Puberty Development in The MINIMat CohortDocument27 pagesActa Paediatrica - 2019 - Svefors - Stunting Recovery From Stunting and Puberty Development in The MINIMat CohortYaumil FauziahNo ratings yet
- Risk Factor Assessment For Pre-Eclampsia: A Case Control StudyDocument6 pagesRisk Factor Assessment For Pre-Eclampsia: A Case Control StudyPutra SeptiansyahNo ratings yet
- Cohort Profile: NICHD Fetal Growth Studies - Singletons and TwinsDocument13 pagesCohort Profile: NICHD Fetal Growth Studies - Singletons and Twinselin luhulimaNo ratings yet
- NIH Public Access: Pregnancy Complications and The Risk of Metabolic Syndrome For The OffspringDocument12 pagesNIH Public Access: Pregnancy Complications and The Risk of Metabolic Syndrome For The OffspringTika Renwarin Tua ElNo ratings yet
- FIGO (International Federation Obstetrics and Gynecology) Pre-Eclampsia 2019Document58 pagesFIGO (International Federation Obstetrics and Gynecology) Pre-Eclampsia 2019Haykal Estu BhismoroNo ratings yet
- Pregnancy and Child Health Outcomes in Pediatric and Young Adult Leukemia and Lymphoma Survivors: A Systematic ReviewDocument25 pagesPregnancy and Child Health Outcomes in Pediatric and Young Adult Leukemia and Lymphoma Survivors: A Systematic ReviewAbdurrahman HasanuddinNo ratings yet
- Influence of Maternal Obesity On The Long-Term Health of OffspringDocument12 pagesInfluence of Maternal Obesity On The Long-Term Health of OffspringAndhika DNo ratings yet
- 1993 - BARKER Et Al - Fetal Nutrition and Cardiovascular Disease in Adult LifeDocument4 pages1993 - BARKER Et Al - Fetal Nutrition and Cardiovascular Disease in Adult LifeSamanta MonteiroNo ratings yet
- The Complex Aetiology of Cerebral PalsyDocument16 pagesThe Complex Aetiology of Cerebral PalsyAileen MéndezNo ratings yet
- Maternal Obesity 2Document13 pagesMaternal Obesity 2lalimdNo ratings yet
- Short Term and Long Term EffectsDocument9 pagesShort Term and Long Term EffectsmariapaulapmNo ratings yet
- Yajnik 2014Document11 pagesYajnik 2014Hashem Essa QatawnehNo ratings yet
- Progesterone To Prevent Spontaneous Preterm BirthDocument28 pagesProgesterone To Prevent Spontaneous Preterm BirthMiriam Rebeca Wills ThomasNo ratings yet
- Association Between Childhood Obesity and Later Life Kidney Disorders: A Systematic ReviewDocument9 pagesAssociation Between Childhood Obesity and Later Life Kidney Disorders: A Systematic ReviewMelinda Wahyu PutriNo ratings yet
- Hypertensive Disorders of Pregnancy: Case Definitions & Guidelines For Data Collection, Analysis, and Presentation of Immunization Safety DataDocument8 pagesHypertensive Disorders of Pregnancy: Case Definitions & Guidelines For Data Collection, Analysis, and Presentation of Immunization Safety DataRachmad SammuliaNo ratings yet
- Nutrients 13 04176Document20 pagesNutrients 13 04176priskylaNo ratings yet
- Adolescent Preeclampsia: Pathological Drivers and Clinical Prevention StrategiesDocument13 pagesAdolescent Preeclampsia: Pathological Drivers and Clinical Prevention StrategiesEmmanuel Guevara HernándezNo ratings yet
- Barker 2009Document14 pagesBarker 2009A. Mukramin YusufNo ratings yet
- Hypospadias and Early Gestation Growth Restriction in InfantsDocument8 pagesHypospadias and Early Gestation Growth Restriction in InfantsNaveed HussainNo ratings yet
- The obstetrician's role in preventing long-term cardiometabolic diseaseDocument8 pagesThe obstetrician's role in preventing long-term cardiometabolic diseaseKeeranmayeeishraNo ratings yet
- AbruptionDocument13 pagesAbruptionMauricio Lopez MejiaNo ratings yet
- Dr. Dr. Nugrahanti - PITFM23 PDFDocument24 pagesDr. Dr. Nugrahanti - PITFM23 PDFDarameutia obgynNo ratings yet
- Breast Milk Consumption in Preterm Neonates and Cardiac Shape in AdulthoodDocument29 pagesBreast Milk Consumption in Preterm Neonates and Cardiac Shape in AdulthoodAsyha KantifaNo ratings yet
- O Papel Da Nutrição Infantil Na Epidemia Global de Doenças Não TransmissiveisDocument7 pagesO Papel Da Nutrição Infantil Na Epidemia Global de Doenças Não TransmissiveisGlauciaNo ratings yet
- Davies 2016Document9 pagesDavies 2016Agung GuretnoNo ratings yet
- Godfrey2000 Godfrey, K. M., & Barker, D. J. (2000) - Fetal Nutrition and Adult Disease.Document9 pagesGodfrey2000 Godfrey, K. M., & Barker, D. J. (2000) - Fetal Nutrition and Adult Disease.Nancy Aidée Reyes MéndezNo ratings yet
- Origins of Adolescent Obesity and Hypertension: SciencedirectDocument2 pagesOrigins of Adolescent Obesity and Hypertension: SciencedirectAurnha DewhiNo ratings yet
- Acta Obstet Gynecol Scand - 2023 - McNestry - Pregnancy Complications and Later Life Women S HealthDocument9 pagesActa Obstet Gynecol Scand - 2023 - McNestry - Pregnancy Complications and Later Life Women S Healthnawal asmadiNo ratings yet
- Article 7 (W7)Document11 pagesArticle 7 (W7)ayaa222nbNo ratings yet
- (Clinical Gastroenterology) Michelle Rook, Philip Rosenthal (Auth.), Maureen M. Jonas (Eds.) - Viral Hepatitis in Children - Unique Features and Opportunities (2010, Humana Press)Document176 pages(Clinical Gastroenterology) Michelle Rook, Philip Rosenthal (Auth.), Maureen M. Jonas (Eds.) - Viral Hepatitis in Children - Unique Features and Opportunities (2010, Humana Press)Mădălina SuciuNo ratings yet
- Jurnal Abrupsio PlasentaDocument13 pagesJurnal Abrupsio Plasentaperussi pranadiptaNo ratings yet
- Preclampsia Lancet 2016Document13 pagesPreclampsia Lancet 2016Irving HernandezNo ratings yet
- Vol9 Issue1 06Document4 pagesVol9 Issue1 06annisaNo ratings yet
- Maternal Obesity and Diabetes Mellitus As Risk Factors For Congenital Heart Disease in The OffspringDocument9 pagesMaternal Obesity and Diabetes Mellitus As Risk Factors For Congenital Heart Disease in The OffspringBianca CaterinalisendraNo ratings yet
- 17 Periodontal Disease - JIOH-4Document6 pages17 Periodontal Disease - JIOH-4Nami RajpootNo ratings yet
- Early Nutrition Programming of Long Term HealthDocument8 pagesEarly Nutrition Programming of Long Term HealthYến Hằng TrầnNo ratings yet
- Genetics of childhood obesity and high-throughput DNA sequencingDocument45 pagesGenetics of childhood obesity and high-throughput DNA sequencingvisiniNo ratings yet
- Being Macrosomic at Birth Is An Independent Predictor of Overweight in Children: Results From The IDEFICS StudyDocument9 pagesBeing Macrosomic at Birth Is An Independent Predictor of Overweight in Children: Results From The IDEFICS StudySayuri BenitesNo ratings yet
- CHD and MalnutDocument9 pagesCHD and MalnutMuhammad AfifNo ratings yet
- Standards Antenatal CareDocument2 pagesStandards Antenatal CareponekNo ratings yet
- Nihms 1806846Document21 pagesNihms 1806846mynana 222No ratings yet
- Preeclampsia An Obstetrician's Perspective - XackdDocument10 pagesPreeclampsia An Obstetrician's Perspective - XackdLiz Valentina Jordan MoyaNo ratings yet
- Phenotype-Directed Management of Hypertension in Pregnancy: Contemporary ReviewDocument18 pagesPhenotype-Directed Management of Hypertension in Pregnancy: Contemporary ReviewElmer MoscosoNo ratings yet
- The Interactive Effect of Prepregnancy Overweight/Obesity and Isolated Maternal Hypothyroxinemia On MacrosomiaDocument8 pagesThe Interactive Effect of Prepregnancy Overweight/Obesity and Isolated Maternal Hypothyroxinemia On MacrosomiaAndres GallegosNo ratings yet
- Prevalence and Determinants of Anemia in Pregnancy, Sana'a, YemenDocument8 pagesPrevalence and Determinants of Anemia in Pregnancy, Sana'a, YemenIJPHSNo ratings yet
- Prevention of Preterm LabDocument6 pagesPrevention of Preterm LabDanTe D' WinchesterNo ratings yet
- Black DKK, 2013Document25 pagesBlack DKK, 2013Yurina HirateNo ratings yet
- Lancet Neurol 2015 - p92Document11 pagesLancet Neurol 2015 - p92astriNo ratings yet
- Pregnancy and Childbirth Outcomes Among Adolescent Mothers: A World Health Organization Multicountry StudyDocument9 pagesPregnancy and Childbirth Outcomes Among Adolescent Mothers: A World Health Organization Multicountry StudyMahyudin SimarokoNo ratings yet
- Toda InfoDocument11 pagesToda InfoGabriel Guillen AsencioNo ratings yet
- Anemia-1Document15 pagesAnemia-1Intan Wahyu CahyaniNo ratings yet
- E058068 FullDocument7 pagesE058068 FullJordan HutabaratNo ratings yet
- Restrictia de Crestere FetalaDocument7 pagesRestrictia de Crestere FetalaBianca MariaNo ratings yet
- Childhood Antecedents To Adult Cardiovascular Disease (PedRev2012)Document13 pagesChildhood Antecedents To Adult Cardiovascular Disease (PedRev2012)jose matosNo ratings yet
- NIH Public Access: Placental Histomorphometry in Gestational Diabetes MellitusDocument13 pagesNIH Public Access: Placental Histomorphometry in Gestational Diabetes MellitusMayra PereiraNo ratings yet
- Overweight in Children and Adolescents: AHA Scientific StatementDocument14 pagesOverweight in Children and Adolescents: AHA Scientific StatementIvan HeriawanNo ratings yet
- CDC 84627 DS1Document15 pagesCDC 84627 DS1Thu HoaiNo ratings yet
- 1 s2.0 S2666668520300495 MainDocument7 pages1 s2.0 S2666668520300495 MainHadikagusti AdoraNo ratings yet
- GFR RBF Tubular in Infancy 1948Document9 pagesGFR RBF Tubular in Infancy 1948Nia Prajnya SyailendraNo ratings yet
- Short Term Gestation and Long Term Risk. Premature CKD Pediatrics.Document14 pagesShort Term Gestation and Long Term Risk. Premature CKD Pediatrics.Nia Prajnya SyailendraNo ratings yet
- Gending Fashion ShowDocument1 pageGending Fashion ShowNia Prajnya SyailendraNo ratings yet
- International Paediatric Neonatal CongressDocument8 pagesInternational Paediatric Neonatal CongressNia Prajnya SyailendraNo ratings yet
- Perlindungan Hukum Terhadap Tenaga Kesehatan Dalam Melaksanakan Tugas Dan Profesinya PDFDocument2 pagesPerlindungan Hukum Terhadap Tenaga Kesehatan Dalam Melaksanakan Tugas Dan Profesinya PDFAnonymous erQHDExTNo ratings yet
- Recommended Childhood Vaccine ScheduleDocument8 pagesRecommended Childhood Vaccine ScheduleDimitris TasiouNo ratings yet
- Patient Focus Therapies On AsthmaDocument12 pagesPatient Focus Therapies On AsthmaNia Prajnya SyailendraNo ratings yet
- Assessment For Nutrition Lecture - 15thoct2013rikaDocument3 pagesAssessment For Nutrition Lecture - 15thoct2013rikaNia Prajnya SyailendraNo ratings yet
- Commercial Cage Farming of Grouper in IndonesiaDocument23 pagesCommercial Cage Farming of Grouper in IndonesiaNia Prajnya SyailendraNo ratings yet
- 6santi - Sulandari - Volume 11 No. 1 Mei 2009Document11 pages6santi - Sulandari - Volume 11 No. 1 Mei 2009Nia Prajnya SyailendraNo ratings yet
- GROUPER Medan WorkshopDocument232 pagesGROUPER Medan WorkshopNia Prajnya SyailendraNo ratings yet
- Doraemon Sheet MusicDocument5 pagesDoraemon Sheet Musicmykitty93% (14)
- Screening of Rop in RSCM, Adriono, 2006Document6 pagesScreening of Rop in RSCM, Adriono, 2006Nia Prajnya SyailendraNo ratings yet
- Assessment For Nutrition Lecture - 15thoct2013rikaDocument3 pagesAssessment For Nutrition Lecture - 15thoct2013rikaNia Prajnya SyailendraNo ratings yet
- Rapid Assessment of Avoidable Blindness - Hans Limburg - 16sept2013Document22 pagesRapid Assessment of Avoidable Blindness - Hans Limburg - 16sept2013Nia Prajnya Syailendra100% (1)
- RoP Epidemics, Incidence, Prevalence, Blindness, Slovakia, 2010Document4 pagesRoP Epidemics, Incidence, Prevalence, Blindness, Slovakia, 2010Nia Prajnya SyailendraNo ratings yet
- Skema ObesDocument1 pageSkema ObesNia Prajnya SyailendraNo ratings yet
- Table 4.2 and 4.3 q1Document4 pagesTable 4.2 and 4.3 q1JOVEMEA LIRAYNo ratings yet
- Acupuncture Points For The TreatmentDocument8 pagesAcupuncture Points For The TreatmentBahtiar Al FikriNo ratings yet
- IB HL Biology Chapter 2 Notes CellsDocument5 pagesIB HL Biology Chapter 2 Notes CellsTiffani Choy100% (1)
- Interpretation of Head CT Scan Ischaemic StrokeDocument7 pagesInterpretation of Head CT Scan Ischaemic StrokePutra DhyatmikaNo ratings yet
- Fehling's Test: Comparative Test Reactions of CarbohydratesDocument33 pagesFehling's Test: Comparative Test Reactions of CarbohydratesTom Anthony Tonguia100% (1)
- Erythritol-Sucralose As Replacement For SugarDocument4 pagesErythritol-Sucralose As Replacement For SugarlpuntyNo ratings yet
- Case Study Number 1Document5 pagesCase Study Number 1Kevin Kyle RizarriNo ratings yet
- The Incredible EGGDocument58 pagesThe Incredible EGGGrowel Agrovet Private Limited.No ratings yet
- Eating Disorders - CausesDocument2 pagesEating Disorders - Causes19PSY05 ATULYA VENKATESHNo ratings yet
- Low Back Pain-Orthoinfo - AaosDocument13 pagesLow Back Pain-Orthoinfo - AaosBang mantoNo ratings yet
- Effect of Cinnamon Decoction on Fasting Blood Sugar LevelsDocument8 pagesEffect of Cinnamon Decoction on Fasting Blood Sugar LevelswantiNo ratings yet
- Group 7 - McDonald Case StudyDocument28 pagesGroup 7 - McDonald Case StudyNhư HảoNo ratings yet
- Relaying Event Details OrallyDocument6 pagesRelaying Event Details OrallyMinson SimNo ratings yet
- Soaps: ContentsDocument13 pagesSoaps: ContentsYaadeshkumaarNo ratings yet
- 6131 01 MSC 20080610Document16 pages6131 01 MSC 20080610Prince AhmedNo ratings yet
- 20 Apple Cider Vinegar Uses + 6 Health Benefits Explained in DetailDocument17 pages20 Apple Cider Vinegar Uses + 6 Health Benefits Explained in DetailAnjar WijayadiNo ratings yet
- How Nutrition and Sleep Impact Each OtherDocument14 pagesHow Nutrition and Sleep Impact Each OtherMonty SharmaNo ratings yet
- FAQ Updated Must Read For Apple Therapy-9Document23 pagesFAQ Updated Must Read For Apple Therapy-9dpparkheNo ratings yet
- Chapters1 5Document75 pagesChapters1 5api-3722454100% (2)
- Calorie Needs by Age, Gender and ActivityDocument2 pagesCalorie Needs by Age, Gender and ActivityИндийскаяДевушкаNo ratings yet
- Flavoured Milk Market ReportDocument12 pagesFlavoured Milk Market ReportKaren NoronhaNo ratings yet
- SBFP Master ListDocument28 pagesSBFP Master ListReymart Tandang AdaNo ratings yet
- Nagata: Chair Scales With Wheel & Bmi ReadingDocument1 pageNagata: Chair Scales With Wheel & Bmi ReadingEong Huat Corporation Sdn BhdNo ratings yet
- Drew Hunter's Favorite Workouts and Example Quality SessionDocument14 pagesDrew Hunter's Favorite Workouts and Example Quality Sessionaman dhillonNo ratings yet
- Physical Activity and Nutrition GuideDocument13 pagesPhysical Activity and Nutrition GuideJen TeruelNo ratings yet
- Mi 40 X Pro AdvancedDocument49 pagesMi 40 X Pro Advancedkrymis0% (1)
- 2019 ESC/EAS Guidelines For The Management of Dyslipidaemias: Lipid Modification To Reduce Cardiovascular Risk: Supplementary DataDocument18 pages2019 ESC/EAS Guidelines For The Management of Dyslipidaemias: Lipid Modification To Reduce Cardiovascular Risk: Supplementary DataElena CosminaNo ratings yet
- The Ideal WeightDocument6 pagesThe Ideal WeightmoB0BNo ratings yet
- ADOLESCENT Obesity MXDocument55 pagesADOLESCENT Obesity MXNor HidayahNo ratings yet
- Government Intervention Needed to Curb Obesity EpidemicDocument2 pagesGovernment Intervention Needed to Curb Obesity Epidemicshugo charaxxx100% (1)