Professional Documents
Culture Documents
PART 1 nCHAPTER 9-TRANS
Uploaded by
Quevee Condez0 ratings0% found this document useful (0 votes)
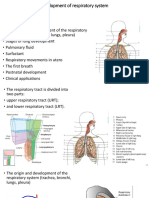
3 views7 pagesThe document summarizes the development of the respiratory system from embryonic stages through birth. It describes that respiratory development begins in the embryonic period around week 5 with the formation of the trachea and lungs. Between weeks 6-16, the lung undergoes pseudoglandular development with growth and branching of the tracheobronchial tree. From weeks 16-26, the lung is in the canalicular stage with further development of respiratory bronchioles. Near term from weeks 32-40, the lung completes alveolar development and is capable of gas exchange at birth.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document summarizes the development of the respiratory system from embryonic stages through birth. It describes that respiratory development begins in the embryonic period around week 5 with the formation of the trachea and lungs. Between weeks 6-16, the lung undergoes pseudoglandular development with growth and branching of the tracheobronchial tree. From weeks 16-26, the lung is in the canalicular stage with further development of respiratory bronchioles. Near term from weeks 32-40, the lung completes alveolar development and is capable of gas exchange at birth.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
3 views7 pagesPART 1 nCHAPTER 9-TRANS
Uploaded by
Quevee CondezThe document summarizes the development of the respiratory system from embryonic stages through birth. It describes that respiratory development begins in the embryonic period around week 5 with the formation of the trachea and lungs. Between weeks 6-16, the lung undergoes pseudoglandular development with growth and branching of the tracheobronchial tree. From weeks 16-26, the lung is in the canalicular stage with further development of respiratory bronchioles. Near term from weeks 32-40, the lung completes alveolar development and is capable of gas exchange at birth.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 7
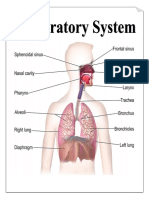
EGAN’S FUNDAMENTALS OF RESPIRATORY THERAPY
CHAPTER 9: THE RESPIRATORY SYSTEM
REVIEWER – Neonatal and Pediatric
• Respiratory development BEGINS in
PRIMARY FUNCTION of the Respiratory EMBRONIC PERIOD: approximately
System: Gas exchange and continuous day 22 after fertilization
absorption of oxygen and elimination of carbon ❖ primitive laryngotracheal tube
dioxide forms from a groove in the
fourth pharyngeal pouch
• External respiration- exchange ❖ From that groove a tracheal
between alveolar gas and blood bud forms by the end of the
• Internal respiration- exchange of gas fourth week of life
between blood and tissues at the ❖ WEEK 5: tracheal bud
cellular level continues to develop and
Close “match” of gas and blood across a large bifurcates into left and right
but extremely thin blood-gas barrier membrane primary bronchial buds
enables efficient gas exchange to occur via • Approximately 6 WEEKS: lung and
simple diffusion airway growth has the appearance of a
glandular structure (6 to 16 weeks)
• Approximately 250 million liters of PSEUDOGLANDULAR STAGE
each are moved and matched during ❖ NEXT 10 WEEKS: growth and
an average 75-year life span. branching of the
• Respiratory system is regulated by the tracheobronchial tree and
nervous system: It is capable of pulmonary vasculature
increasing function in response to continue and culminate with
elevated demands brought on by formation of the terminal
stressful conditions such as exercise and respiratory bronchioles.
and disease ▪ TERMINAL
BRONCHIOLES:
conducting airways only
DEVELOPMENT OF THE RESPIRATORY SYSTEM and do not participate in
gas exchange with blood
▪ RESPIRATORY
• Developmental phases of a fertilized BRONCHIOLES: more
egg are divided into the embryonic superficial capillaries
and fetal periods. and are capable of gas
❖ EMBRYONIC - first 8 weeks exchange with blood,
of pregnancy; Major organs becoming more
will develop elaborate as
❖ FETAL - remaining 32 weeks development continues
of pregnancy; organs continue ❖ Epithelial lining (airways)
▪ Columnar epithelia-
to develop and refine their
proximal airways
structure and function ▪ Cuboidal epithelia-
THREE DISTINCT GERMINAL TISSUE more distal bronchioles
• WEEK 16-26:
LAYERS: endoderm, mesoderm, and
ectoderm CANALICULAR STAGE
❖ overlaps with the
Pseudoglandular stage because
the superior regions are
developing slightly faster than the
inferior regions
❖ PRIMARY CHANGES…………...
Development of two to four more
generations of respiratory
bronchioles from each terminal
bronchiole.
❖ Last several weeks: region
beyond each terminal bronchiole
forms the functional structure –
EGAN’S FUNDAMENTALS OF RESPIRATORY THERAPY
CHAPTER 9: THE RESPIRATORY SYSTEM
REVIEWER – Neonatal and Pediatric
• 32nd- 40th WEEK- ALVEOLAR STAGE
❖ ACINUS- Basic gas-exchange
unit of the lung ❖ development of mature
❖ Two principal epithelial cell types alveoli, accompanied by
-cover the gas-exchange surface capillary proliferation within
(begin to appear) the walls
▪ Type I Pneumocyte- ❖ FINAL PHASE OF LUNG
FLATTER squamous DEVELOPMENT
epithelial cells ❖ terminal saccules develop
▪ Type II Pneumocyte -
hexagonal pouchlike region –
ROUNDED secretory
ALVEOLI
cells
❖ At the end of the canalicular
period (24 to 26 weeks of
gestation), the fetus, if born, is
capable of sufficient gas
exchange and viable if supported
with supplemental O2, ventilatory
support, and surfactant
administration
❖ FULL TERM NEW BORN
• 26 weeks to birth - TERMINAL
INFANT: 50 MILLION
SACCULAR STAGE
❖ more terminal bronchioles and their alveoli, this number
associated acini form and develop continues to increase for
❖ COMPLETE formation of the total approximately 2 to 3 years
number of terminal bronchioles after birth
❖ Differentiate Type I and II ❖ PRODUCED AROUND 24
Pneumocytes TO 25 WEEKS of
❖ Capillaries continue to form near and development by type II
bulge from the surface of the acinus pneumocytes --
Pulmonary Surfactant-
promotes lung inflation
and protects the alveolar
surface
❖ PS: phospholipids, small
amount of protein (types SP-
A, SP-B, and SP-C), and a
trace of carbohydrates
❖ Phospholipid
components:
o phosphatidylcholine
(lecithin [L] and
sphingomyelin [S])
o phosphatidylglycerol
(PG).
❖ Type I Pneumocytes – ❖ The amount of these
thin and elongate to cover phospholipids (the L/S
saccule walls -- primary gas- ratio and PG
exchange cells in lung with concentration) provides a
close approximation to predictive index of the
developing pulmonary lung maturity in a fetus
capillaries before birth and the risks
❖ Type II Pneumocytes- for the development of
form and secrete the vital
respiratory distress
pulmonary surfactants that
are necessary to alter ❖ L/S Ratio:
surface tension and help o ≥2 - Relatively LOW
keep the lungs inflated risk
o <1.5 – HIGH Risk
EGAN’S FUNDAMENTALS OF RESPIRATORY THERAPY
CHAPTER 9: THE RESPIRATORY SYSTEM
REVIEWER – Neonatal and Pediatric
• Surfactant synthesis: regulated by
hormones, genes and factors including TRANSITION FROM UTERINE TO
GLUCOCORTICOIDS EXTRAUTERINE LIFE
• GLUCOCORTICOSTEROID
PRODUCTION- increases at the end of PLACENTAL STRUCTURE AND FUNCTION
gestation and stimulates receptors in type • PLACENTA- provide fetus effective
II pneumocytes, increasing surfactant circulatory interface with the circulation of
production and improving the L/S ratio. the mother
• DISTINCTIVE function of the • 1 WEEK (Uterine Implantation)
developing lung: formation of relatively ❖ Chorionic villi- vascular
large amounts of fetal lung fluid that passes projections that arise from
into amniotic fluid. chorion of embryo ► penetrate
• Fetal lung fluid: plasma ultrafiltrate from fetal the uterine endometrium
pulmonary microcirculation + components of ❖ Gestation proceeds > villi
pulmonary surfactant + other fluids from pulmonary increase in number and
epithelial cells
complexity > erode the
• CONSTANTLY PRODUCED AND
endometrium > create regular
REPLACE – keeping fetal lung inflated at
pockets (INTERVILLOUS
slight positive pressure with respect to
SPACES) --- filled with maternal
amniotic fluid pressure
blood
• IMPORTANT in stimulating normal lung
▪ Maternal blood in
development
intervillous spaces
• At term- fetal lung filled with bathes embryonic villi
approximately 40 mL of fluid creating oxygen-rich
• incomplete inflation and poorly and nutrient-rich
developed (hypoplastic) lungs ► environment
inadequate fetal breathing and low • Maternal blood flows into the intervillous space
amounts of amniotic fluid formation through the spiral arteries, whereas fetal blood
(oligohydramnios) is supplied to the villi from two umbilical
• MID GESTATION: fetus begins to make arteries.
respiratory efforts with little to no fluid in • Maternal and fetal blood come into close
and out of the lungs proximity but remain separated by an
• RHYTHM AND DEPTH: Periodic and embryonic membrane
Irregular (reflecting the development of • Oxygenated fetal blood – LEAVES
respiratory centers in the brain and chorionic villi capillaries through placental
respiratory muscles) venules >> RETURNS to the fetus through
• At birth SINGLE UMBILICAL VEIN
❖ Lungs of MALE infant: larger +
have greater number of
respiratory bronchioles
❖ Evaluating breathing efforts and
surfactant production: 26-36
WEEKS of gestation – FEMALE
have better developed lung
function + slightly less susceptible
to RDS
EGAN’S FUNDAMENTALS OF RESPIRATORY THERAPY
CHAPTER 9: THE RESPIRATORY SYSTEM
REVIEWER – Neonatal and Pediatric
• P50 (PO2 that saturates 50% of
• STUNT intrauterine growth – abnormal the hemoglobin) -- 6 to 8 mm
implantation of the placenta, tearing of Hg less than the P50 for adult
the placenta from the uterine wall, or hemoglobin (HbA) -- indicates
decreased placental blood flow ► fetal the degree of the shift toward
asphyxia, increase risk for brain higher affinity
damage, respiratory distress in • AT BIRTH - 70% of circulating
immediate post-natal period hemoglobin is HbF
• HbA gradually replaces HbF
during the first 6 months of
extrauterine life ► HbA genes in
bone marrow switch on and
HbF genes in the liver (major
site of fetal erythrocyte
development) are switched off
\
FETAL CIRCULATION
• Assessment of umbilical vein blood
gas data (cord blood gas) shortly after
birth is a method of determining the
degree of fetal asphyxiation during the
birth process
• O2 content and delivery by fetal blood
are almost the same as adult blood
despite the much lower PO2
❖ FACTORS: in fetal blood
1. Relatively higher
content of hemoglobin
(18 g/dL)
2. Hematocrit (54%)
3. Presence of fetal
hemoglobin • Three important BYPASS pathways
✓ increased affinity
(shunts)
for O2 and a more
pronounced Bohr • ductus venosus, ductus
effect (reduced arteriosus, and foramen ovale
oxyhemoglobin • Approximately ONE-THIRD of
affinity with OXYGENATED blood flows to the lower
acidosis) to trunk and extremities
enhance O2 • other TWO-THIRDS flows through the
release ductus venosus, bypassing the liver’s
circulation, and flows to the inferior vena
cava
• This BETTER OXYGENATED BLOOD in
the inferior vena cava mixes with the
venous blood returning from the lower
trunk and extremities entering the right
atrium
• APPROXIMATELY 50% -- (of this blood)
is shunted from the right atrium into the
left atrium through an opening in the
interatrial septum called the foramen
ovale
• Left atrial blood flows to the left ventricle
and then to the ascending aorta, where it
continues on to the brain, brachiocephalic
trunk, and descending aorta.
EGAN’S FUNDAMENTALS OF RESPIRATORY THERAPY
CHAPTER 9: THE RESPIRATORY SYSTEM
REVIEWER – Neonatal and Pediatric
• VENOUS BLOOD from the Superior Vena
Cava is directed downward through the • Normal Vaginal Delivery
right atrium into the right ventricle and then o 1/3 is cleared through the
into the main pulmonary artery compression of the thorax
• DUCTUS ARTERIOSUS (a muscular o 2/3 cleared through pulmonary
vessel attached to the trunk of the capillaries and lymphatics
pulmonary artery and the aorta) – DILATE • Must develop very high transpulmonary
+ Pulmonary Arteries CONSTRICT ------ pressure -- overcome opposing forces of
due to relatively low PO2 and various fluid viscosity in the airways and surface
prostaglandins in fetal blood tension of the alveoli
• Leads to INCREASE IN PULMONARY • Stimuli via peripheral and central
VASCULAR RESISTANCE = pulmonary chemoreceptors augmented by skin
artery pressure higher than aortic blood receptors
pressure • FIRST BREATH – new tactile and thermal
• RESULT – 90% of blood flow entering the stimuli
pulmonary artery takes the path of least • First, no air enters newborn lung until
resistance by shunting through the ductus transpulmonary pressure exceeds 40
arteriosus to aorta cmH20
• ONLY 10% - flows into the LUNGS • As volume increases, amount of pressure
• Blood from Ductus Arteriosus + Blood from decreases
aorta → systemic circulation (Some of this • As lung filled with air – results in
blood flows to the gut, lower extremities, and pulmonary vasodilation
placenta) o pH increases
• TWO UMBILICAL ARTERIES – Carry blood
o PO2 increases
from fetal aorta to the placenta (carrying out
o PCO2 decreases
fetal-maternal gas and nutrient exchange)
Result:
♣ Pulmonary Vasodilation
♣ Lower pulmonary vascular resistance
♣ Constriction of the Ductus Arteriosus
Greater blood flow through pulmonary
circulation
• Maternal loss of prostaglandin – closure of
ductus arteriosus
• Alveolar air content + constriction =
promotes matching of ventilation and blood
flow
• Clamping of umbilical cord – cessation of
umbilical and placental blood flow =
closure of ductus venosus and increase
Systemic vascular resistance
• ↑ Systemic Vascular Resistance = ↑ left
sided heart pressure = ↑ Left atrial
pressure = Closes Foramen ovale
• Anatomic closure of the ductus normally
occurs within 3 weeks of birth.
Permanent closure of the tissue flap
covering the foramen ovale may take
several months
• Days before birth, the epithelia of the lung
stop the production of lung fluid –
absorbed back into the fetal circulation
EGAN’S FUNDAMENTALS OF RESPIRATORY THERAPY
CHAPTER 9: THE RESPIRATORY SYSTEM
REVIEWER – Neonatal and Pediatric
• Anatomic deadspace is smaller at
approx. 1.5 ml/kg of bodyweight
• Mainstem bronchi – branch off from
POSTNATAL LUNG DEVELOPMENT trachea at less acute angle
o Right mainstem bronchus is more
UPPER AIRWAY align with trachea
• Mean airway diameter – main bronchi to
respiratory bronchioles -- ↑ 2x – 3x
• Smooth muscle (airways to respiratory
bronchioles) increases until 8 months
• Distinct C-shaped rings of cartilage are
found in the trachea and main stem
bronchi of the neonate
• Increase likelihood of airway obstruction
o Greater relative weight of head –
acute flexion of cervical spine –
infant neck flexion
o Smaller nasal passages than
adult
o Jaw – rounder
o Tongue – much larger relative to
size of oral cavity
LOWER AIRWAY AND ALVEOLI
• Most infants prefer to breathe through
• 480 million alveoli
nose
• All development is generally complete by
• Shift to oral breathing through nasal
10 years of age – most occurring at 1 ½
occlusion and hypoxia
post-natal year
• 4 to 5 months of age – full oral ventilation
• Adulthood – ACM gas exchange surface
• Larynx – higher in the neck – GLOTTIS area 140m2
located between C3 and C4, more funnel
• Gene and mechanical stretch → STEM
shaped
CELL ACTIVATION IN THE LUNGS –
o Swallowing - larynx provides a
responsible for alveolar development in
direct connection to the
adulthood after loss of lung tissue
nasopharynx- Allow breathe and
suckle at the same time DEVELOPMENT OF THE VASCULAR,
• Narrowest region of upper airway – LYMPHATIC, AND NERVOUS SYSTEM
cricoid cartilage • The respiratory system is a unique organ
o Glottis in adult in that it receives a double blood supply:
• Epiglottis – longer and less flexible, lies o one from the left ventricle and
higher and more horizontal o one from the right ventricle
• Anatomic descent of the epiglottis begins • right heart – bulk of flow to pulmonary
at 2 1/2 to 3 months of age. circulation
• Large conducting airways – shorter and • left heart – smaller amount of flow (1% -
narrower 2%)
• Trachea • Bronchial arteries – supply O2 to the
o normal newborn airway tissue, blood vessels, nerves,
▪ 5-6 cm long & 4mm lymphatics, and visceral pleura
diameter • The lung’s double circulation benefits
o Small preterm the entire lung in health and helps
▪ 2 cm long & 2-3 mm compensate for deficiencies or disease
diameter processes that can affect either circulation.
EGAN’S FUNDAMENTALS OF RESPIRATORY THERAPY
CHAPTER 9: THE RESPIRATORY SYSTEM
REVIEWER – Neonatal and Pediatric
• Lymphatic vessels – central role in the
control of fluid and protein balance
within the lung and house various
defensive cells
• Fluid collected from the pleural space and
interstitium is carried by the pleural
capillaries and vessels through the
lymphatic system back to the root of the
lung (hilum), where numerous lymph
nodes are located
• Neuronal centers in brainstem – medulla
oblongata and pons
o Automatic control of breathing
• Phrenic and intercostal nerves – primary
component of somatic (motor) nervous
system that carry nervous signals from
brainstem to respiratory muscles
o Diaphragm – phrenic nerves
o Intercostal muscles – intercostal
nerves
o responsible for enlarging the
thorax (inspiration) and allow
exhalation by relaxing, letting the
thorax and lungs recoil back to
their preinspiratory position
• Sympathetic and Parasympathetic –
visceral control of the smooth muscle of
respiratory system
o Sympathetic
▪ Bronchodilation of
bronchioles
▪ Vasoconstriction of
blood vessels
o Parasympathetic
▪ Mucus glands to
produce mucus
• Cranial Nerve X (Vagus Nerve) – carries
motor and sensory signals of
parasympathetic system
• Branches from each thoracic spinal
nerve carry sympathetic motor and
sensory signals to and from the lungs
CHEST WALL DEVELOPMENT, DIAPHRAGM,
AND LUNG VOLUME
• Infant thoracic cage – more box-like with
ribs being horizontally oriented or elevated
• Ossification of the ribs and sternum
normally complete by 25 years of age
• Grunting – “laryngeal braking”
o Narrowing of glottis or larynx
during exhalation
o Infants in respiratory distress
grunt, a manifestation of laryngeal
braking
You might also like
- Essentials of Respiratory SystemDocument138 pagesEssentials of Respiratory SystemMuhammad Ahmad bin makruf syammakuNo ratings yet
- Pedia Pulmo 1Document129 pagesPedia Pulmo 1Sven OrdanzaNo ratings yet
- Respiratory, USMLE ENDPOINT BY DR AHMED SHEBLDocument91 pagesRespiratory, USMLE ENDPOINT BY DR AHMED SHEBLDaNy Chiriac100% (3)
- Embryology: Lung DevelopmentDocument91 pagesEmbryology: Lung DevelopmentPrarthanaNo ratings yet
- Human BodyDocument27 pagesHuman Bodyvigyanashram100% (2)
- Pulmonary EmbolismDocument21 pagesPulmonary EmbolismMadhu Bala100% (2)
- EmbryologyDocument61 pagesEmbryologyAmritha Anand100% (2)
- Measurements in Radiology Made EasyDocument213 pagesMeasurements in Radiology Made EasySllavko K. KallfaNo ratings yet
- Parts & Function of Respiratory SystemDocument4 pagesParts & Function of Respiratory SystemLucille Ballares83% (6)
- The Respiratory System: Supplement To Text, Chapter 9Document77 pagesThe Respiratory System: Supplement To Text, Chapter 9Christina GonezNo ratings yet
- LEsson-Guide-G9-Biology Module 1 On TemplateDocument29 pagesLEsson-Guide-G9-Biology Module 1 On Templateconstancia G, caraan0% (1)
- Breathing Exercise Effective CoughDocument52 pagesBreathing Exercise Effective CoughDesy Suryani Pais100% (1)
- Quintinitarosalyn Module 1-Wk1respiratory SystemDocument35 pagesQuintinitarosalyn Module 1-Wk1respiratory Systemrosalyn quintinita60% (5)
- Coronary Artery DiseaseDocument110 pagesCoronary Artery DiseaseMaesy Garcia LorenaNo ratings yet
- Corticosteroid in Lung MaturationDocument12 pagesCorticosteroid in Lung Maturationtommy japolaNo ratings yet
- 1 (Lung Development)Document12 pages1 (Lung Development)Dr.sonuNo ratings yet
- Intrauterine Development of Respiratory SystemDocument21 pagesIntrauterine Development of Respiratory SystemShubham KathareNo ratings yet
- Development of Respiratory SystemDocument23 pagesDevelopment of Respiratory SystemRas Siko SafoNo ratings yet
- Introduction To Physiological Changes in NEONATAL AND PEDIATRIC RESPICARE James Astrologo LamusaoDocument88 pagesIntroduction To Physiological Changes in NEONATAL AND PEDIATRIC RESPICARE James Astrologo LamusaoDanao, Aira A.No ratings yet
- 20 - Development of Respiratory SystemDocument33 pages20 - Development of Respiratory SystemDr.B.B.GosaiNo ratings yet
- Neonatal Respiratory Care NotesDocument136 pagesNeonatal Respiratory Care NotesJade Louise FkshmNo ratings yet
- 3-Embryology of Respiratory SystemDocument25 pages3-Embryology of Respiratory SystemNur HikmahNo ratings yet
- Respiratory System EmbDocument29 pagesRespiratory System EmbDanish GujjarNo ratings yet
- SEHH2234 2021 S2 Ch14 RespirationDocument56 pagesSEHH2234 2021 S2 Ch14 Respirationmiki leeNo ratings yet
- 12 Lung DevelopmentDocument36 pages12 Lung Developmentf3er3No ratings yet
- Curs C1 Sem 2 Year 1 ENG - Development Respiratory SystemDocument43 pagesCurs C1 Sem 2 Year 1 ENG - Development Respiratory SystemAchraf RbNo ratings yet
- Pedia Pulmo 1Document129 pagesPedia Pulmo 1Sven OrdanzaNo ratings yet
- Pediatric Respiratory PhysiologyDocument15 pagesPediatric Respiratory PhysiologyNica Lopez FernandezNo ratings yet
- Ch. 29 StudyDocument6 pagesCh. 29 StudyPaige Nicole GauthreauxNo ratings yet
- Pediatric and Neonatal Respiratory Care Embryologic DevelopmentDocument205 pagesPediatric and Neonatal Respiratory Care Embryologic DevelopmentAlexander Santiago ParelNo ratings yet
- OxygenationDocument24 pagesOxygenationJohn Anthony de GùzmanNo ratings yet
- Mtap AubfDocument10 pagesMtap AubfMarjorie Balangue MacadaegNo ratings yet
- Development of Respiratory System and Its AnomaliesDocument17 pagesDevelopment of Respiratory System and Its AnomaliesmichaelqurtisNo ratings yet
- Lung Development and Fetal CirculationDocument9 pagesLung Development and Fetal CirculationJade ProvidenceNo ratings yet
- M.03 Respiratory Disorders of Children Part 1 (Dr. Tandoc) (10-26-20)Document7 pagesM.03 Respiratory Disorders of Children Part 1 (Dr. Tandoc) (10-26-20)VanessaNo ratings yet
- Development of Respiratory SystemDocument34 pagesDevelopment of Respiratory SystemNatalie HuiNo ratings yet
- Assessment of The Thorax and LungsDocument9 pagesAssessment of The Thorax and LungsJasmin MolanoNo ratings yet
- All About RespiratoryDocument69 pagesAll About RespiratoryMarcellina Awing100% (1)
- Anatomy Final pt.2Document8 pagesAnatomy Final pt.2Gladys Mae S. BañesNo ratings yet
- OXYGENATIONDocument13 pagesOXYGENATION20233651No ratings yet
- Anatomy Review RespiratoryDocument8 pagesAnatomy Review RespiratoryHanami AsriNo ratings yet
- Chapter 1 - RespirationDocument3 pagesChapter 1 - Respirationqin donNo ratings yet
- RespiratorDocument97 pagesRespiratorandreaNo ratings yet
- 1.1 The Human Respiratory System - 1.2 Gas Exchange - 1.3 BreathingDocument26 pages1.1 The Human Respiratory System - 1.2 Gas Exchange - 1.3 BreathingAla' ShehadehNo ratings yet
- Development of Body Cavities and Diaphragm: Pleuro-Pericardial and Pleuro-Peritoneal MembraneDocument14 pagesDevelopment of Body Cavities and Diaphragm: Pleuro-Pericardial and Pleuro-Peritoneal MembraneÑäd ÉèmNo ratings yet
- Respiratory System: General Histology by Falia, JoyjoyDocument29 pagesRespiratory System: General Histology by Falia, JoyjoyJoyjoy FaliaNo ratings yet
- 0402 Histo LectureDocument46 pages0402 Histo LectureJoe JNo ratings yet
- Reviewer - Rehabilitation of Pulmonary DysfunctionDocument7 pagesReviewer - Rehabilitation of Pulmonary DysfunctionSatria Faye RespicioNo ratings yet
- Bilaminar & Trilaminar DiscsDocument29 pagesBilaminar & Trilaminar DiscsmarktsaihhNo ratings yet
- B5M2C1 REVIEWERDocument31 pagesB5M2C1 REVIEWERMariel AbatayoNo ratings yet
- L10 - Respiratory System ShortenedDocument38 pagesL10 - Respiratory System ShortenedgfsgfdsgfdNo ratings yet
- Embriologi Mata 2015 1Document77 pagesEmbriologi Mata 2015 1yayanNo ratings yet
- Human Development TransesDocument5 pagesHuman Development TransesReign SaplacoNo ratings yet
- Prenatal Growth 1Document64 pagesPrenatal Growth 1Kar InNo ratings yet
- Human Lung DevelopmentDocument20 pagesHuman Lung DevelopmentMila KarmilaNo ratings yet
- Systemic EmbryologyDocument40 pagesSystemic EmbryologyM sarauta TvNo ratings yet
- 1.1 The Human Respiratory System - 1.2 Gas Exchange - 1.3 BreathingDocument26 pages1.1 The Human Respiratory System - 1.2 Gas Exchange - 1.3 BreathingAla' ShehadehNo ratings yet
- Airway Management Lec 1Document4 pagesAirway Management Lec 1Lenard SakiliNo ratings yet
- The Third WeekDocument132 pagesThe Third WeekGeoffreyNo ratings yet
- 25th August Respiratory Integrated Case Study 2020Document44 pages25th August Respiratory Integrated Case Study 2020Sabashnee GovenderNo ratings yet
- Anterior Segment of The EyeDocument48 pagesAnterior Segment of The EyeNadya Beatrix Yohanna NapitupuluNo ratings yet
- 13 - Respiratory SystemDocument68 pages13 - Respiratory SystemNur Shahirah Nasir Faculty of Social Science and FoundationNo ratings yet
- Development of Nervous SystemDocument25 pagesDevelopment of Nervous SystemHemant JoshiNo ratings yet
- Intrauterine Development: Premidterm CoverageDocument14 pagesIntrauterine Development: Premidterm Coverageaidan udjamanNo ratings yet
- Pulmonary Slides 2023 (B&B) (Medicalstudyzone - Com)Document568 pagesPulmonary Slides 2023 (B&B) (Medicalstudyzone - Com)Amna ZafarNo ratings yet
- Gaseous ExchangeDocument27 pagesGaseous Exchangemasibulele641No ratings yet
- Treatise on the Anatomy and Physiology of the Mucous Membranes: With Illustrative Pathological ObservationsFrom EverandTreatise on the Anatomy and Physiology of the Mucous Membranes: With Illustrative Pathological ObservationsNo ratings yet
- Hilar EnlargementDocument19 pagesHilar EnlargementGriggrogGingerNo ratings yet
- A Systematic Approach To The Management of Massive Hemoptysis.Document18 pagesA Systematic Approach To The Management of Massive Hemoptysis.Hitomi-No ratings yet
- Evaluation of Compliance With Standard Criteria For Postero-Anterior (Pa) Chest Radiographs in Parklane Hospital EnuguDocument37 pagesEvaluation of Compliance With Standard Criteria For Postero-Anterior (Pa) Chest Radiographs in Parklane Hospital EnuguLavinia VictorNo ratings yet
- Comparative Anatomy Study of The Respiratory System Between Green Turtle (Chelonia Mydas) and Leatherback Turtle (Dermochelys Coriacea)Document1 pageComparative Anatomy Study of The Respiratory System Between Green Turtle (Chelonia Mydas) and Leatherback Turtle (Dermochelys Coriacea)AldinaNo ratings yet
- Physiotherapy of Avian RespiratoryDocument3 pagesPhysiotherapy of Avian RespiratoryMichael MekhaNo ratings yet
- Benign and Malignant Lesions in Respiratory CytologyDocument43 pagesBenign and Malignant Lesions in Respiratory CytologyfadoNo ratings yet
- Histology of Respiratory SystemDocument6 pagesHistology of Respiratory SystemEzyan Syamin100% (2)
- Nursing Management of Children With Respiratory System DysfunctionsDocument27 pagesNursing Management of Children With Respiratory System DysfunctionsDody ZainNo ratings yet
- Medical Nutrition Therapy For Respiratory DisordersDocument7 pagesMedical Nutrition Therapy For Respiratory DisordersBok MatthewNo ratings yet
- Respiratory SystemDocument13 pagesRespiratory SystemIrica Mae CiervoNo ratings yet
- A Case Study On Pulmonary Tuberculosis: Intensive PracticumDocument36 pagesA Case Study On Pulmonary Tuberculosis: Intensive PracticumJek Dela CruzNo ratings yet
- Grade 9-Summative AssessmentDocument2 pagesGrade 9-Summative AssessmentMaryjoy Piosca MolaNo ratings yet
- Lung Deposition Predictions of Airborne ParticlesDocument10 pagesLung Deposition Predictions of Airborne ParticlesJimmy Wea ChittiwanNo ratings yet
- Respiratory SystemDocument4 pagesRespiratory Systemtarunesh1No ratings yet
- Medical Terminology For Health Professions 6th Edition Ehrlich Test BankDocument9 pagesMedical Terminology For Health Professions 6th Edition Ehrlich Test Banksinapateprear4k100% (34)
- Animal PhysiologyDocument104 pagesAnimal PhysiologyClara MaeNo ratings yet
- Dams CBT 2Document110 pagesDams CBT 2singh2manish100% (2)
- Practice Test 1 WritingDocument4 pagesPractice Test 1 WritingHeywoodNo ratings yet
- Engleski Knjiga - Prvih 10 LekcijaDocument35 pagesEngleski Knjiga - Prvih 10 LekcijaIvanaNo ratings yet
- Class 12 Biology Chapter 14 NotesDocument23 pagesClass 12 Biology Chapter 14 NotesSyed Atta Ur RahmanNo ratings yet
- Physical Examinations Respiratory System: InspectionDocument5 pagesPhysical Examinations Respiratory System: InspectionAzizan HannyNo ratings yet
- Objective Test - 1: TOPIC: 1. Respiratory System 2. Circulatory System MM:50Document6 pagesObjective Test - 1: TOPIC: 1. Respiratory System 2. Circulatory System MM:50Jaskirat SinghNo ratings yet
- Vital SignosDocument5 pagesVital SignosJuan David Vera BohorquezNo ratings yet