Professional Documents
Culture Documents
Chemistry Let Review
Uploaded by
Cawen Tapon0 ratings0% found this document useful (0 votes)
4 views70 pagesThe document contains 20 questions from a chemistry LET review. The questions cover topics like states of matter, chemical and physical changes, molecular geometry, quantum numbers, and molecular polarity. Multiple choice answers are provided for each question.
Original Description:
Original Title
2012402e5750cd17a9a129a98e5c7df0 (1)
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document contains 20 questions from a chemistry LET review. The questions cover topics like states of matter, chemical and physical changes, molecular geometry, quantum numbers, and molecular polarity. Multiple choice answers are provided for each question.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
4 views70 pagesChemistry Let Review
Uploaded by
Cawen TaponThe document contains 20 questions from a chemistry LET review. The questions cover topics like states of matter, chemical and physical changes, molecular geometry, quantum numbers, and molecular polarity. Multiple choice answers are provided for each question.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 70
CHEMISTRY LET REVIEW
1.These particles have the highest kinetic energy
compared to the particles of other states of
matter.
a. Solid
b.Liquid
c. Gas
d.Tincture
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
1.These particles have the highest kinetic energy
compared to the particles of other states of
matter.
a. Solid
b.Liquid
c. Gas
d.Tincture
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
2. The sublimation of albatross deodorizer is an
example of _____.
a. Physical properties
b.Chemical properties
c. Physical changes
d.Chemical changes
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
2. The sublimation of albatross deodorizer is an
example of _____.
a. Physical properties
b.Chemical properties
c. Physical changes
d.Chemical changes
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
3. Solid sodium added to water will produce sodium
hydroxide and hydrogen gas. Formation of hydrogen
gas is an example of _____.
a. Physical properties
b.Chemical properties
c. Physical changes
d.Chemical changes
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
3. Solid sodium added to water will produce sodium
hydroxide and hydrogen gas. Formation of hydrogen
gas is an example of _____.
a. Physical properties
b.Chemical properties
c. Physical changes
d.Chemical changes
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
4. Long exposure to mercury may cause
complications to your nervous, digestive, immune
systems, and at worst, it may cause death. The
property of mercury that can cause these
complications and even death is _____.
a. flammability
b.combustibility
c. toxicity
d.corrosivity Saint Michael College of Caraga
CHEMISTRY LET REVIEW
4. Long exposure to mercury may cause
complications to your nervous, digestive, immune
systems, and at worst, it may cause death. The
property of mercury that can cause these
complications and even death is _____.
a. flammability
b.combustibility
c. toxicity
d.corrosivity Saint Michael College of Caraga
CHEMISTRY LET REVIEW
5. Which of the following separation techniques
would you use to best separate sand from iron
fillings?
a. Bar magnet
b.Distillation apparatus
c. Filter paper
d.Chromatography paper
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
5. Which of the following separation techniques
would you use to best separate sand from iron
fillings?
a. Bar magnet
b.Distillation apparatus
c. Filter paper
d.Chromatography paper
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
6. How many electrons does O-18 have?
a. 8
b.10
c. 16
d.18
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
6. How many electrons does O-18 have?
a. 8
b.10
c. 16
d.18
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
7. How many neutrons does O-18 have?
a. 8
b.10
c. 16
d.18
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
7. How many neutrons does O-18 have?
a. 8
b.10
c. 16
d.18
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
8. What is the net electrical charge of O-18 if it has
10 electrons?
a. 0
b.+2
c. -2
d.+8
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
8. What is the net electrical charge of O-18 if it has
10 electrons?
a. 0
b.+2
c. -2
d.+8
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
9. Name the compound: NaCN
a. Sodium carbon nitride
b.Sodium cyanide
c. Sodium carbon mononitride
d.Sodium carbinitride
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
9. Name the compound: NaCN
a. Sodium carbon nitride
b.Sodium cyanide
c. Sodium carbon mononitride
d.Sodium carbinitride
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
10. What is the name of a molecule that is made up
of a metalloid in Group 3A and three atoms of a
halogen in Period 2.
a. Boron fluoride
b.Silicon chloride
c. Boron trifluoride
d.Silicon trichloride
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
10. What is the name of a molecule that is made up
of a metalloid in Group 3A and three atoms of a
halogen in Period 2.
a. Boron fluoride
b.Silicon chloride
c. Boron trifluoride
d.Silicon trichloride
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
11. One way to remove nitrogen monoxide from
smokestack emissions is to react it with ammonia.
𝑁𝐻3 + 𝑁𝑂 → 𝑁2 + 𝐻2 𝑂
Which of the following is a reactant?
a. Nitrogen monoxide
b.Nitrogen gas
c. Steam
d.Water
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
11. One way to remove nitrogen monoxide from
smokestack emissions is to react it with ammonia.
𝑁𝐻3 + 𝑁𝑂 → 𝑁2 + 𝐻2 𝑂
Which of the following is a reactant?
a. Nitrogen monoxide
b.Nitrogen gas
c. Steam
d.Water
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
12. After balancing the chemical equation shown,
𝑁𝐻3 + 𝑁𝑂 → 𝑁2 + 𝐻2 𝑂
What is the numerical coefficient of nitrogen gas?
a. 1
b.4
c. 5
d.6
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
12. After balancing the chemical equation shown,
𝑁𝐻3 + 𝑁𝑂 → 𝑁2 + 𝐻2 𝑂
What is the numerical coefficient of nitrogen gas?
a. 1
b.4
c. 5
d.6
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
13. In the chemical equation shown,
𝑁𝐻3 + 𝑁𝑂 → 𝑁2 + 𝐻2 𝑂
How many moles of ammonia is needed to produce
6 moles of water?
a. 1
b.4
c. 5
d.6
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
13. In the chemical equation shown,
𝑁𝐻3 + 𝑁𝑂 → 𝑁2 + 𝐻2 𝑂
How many moles of ammonia is needed to produce
6 moles of water?
a. 1
b.4
c. 5
d.6
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
14. What is the formula weight of cyanogen gas,
𝐶2 𝑁2 ? (C = 12g/mol ; N = 14g/mol)
a. 38 g/mol
b.52 g/mol
c. 71 g/mol
d.88 g/mol
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
14. What is the formula weight of cyanogen gas,
𝐶2 𝑁2 ? (C = 12g/mol ; N = 14g/mol)
a. 38 g/mol
b.52 g/mol
c. 71 g/mol
d.88 g/mol
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
15. What is the formula weight of nitrogen
trifluoride, 𝑁𝐹3 ? (N = 14g/mol ; F = 19g/mol)
a. 38 g/mol
b.52 g/mol
c. 71 g/mol
d.88 g/mol
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
15. What is the formula weight of nitrogen
trifluoride, 𝑁𝐹3 ? (N = 14g/mol ; F = 19g/mol)
a. 38 g/mol
b.52 g/mol
c. 71 g/mol
d.88 g/mol
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
16. What will happen to the volume of gas in a
closed and flexible container if the number of moles
of gas is increased?
a. Increase
b.Decrease
c. Remains unchanged
d.Cannot be determined
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
16. What will happen to the volume of gas in a
closed and flexible container if the number of moles
of gas is increased?
a. Increase
b.Decrease
c. Remains unchanged
d.Cannot be determined
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
17. What is the maximum number of electrons can
the 2p orbital hold?
a. 2
b.6
c. 10
d.14
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
17. What is the maximum number of electrons can
the 2p orbital hold?
a. 2
b.6
c. 10
d.14
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
18. Which among the sets of quantum numbers is
valid and an electron can be found?
a. 𝑛 = 3; 𝑙 = 2; 𝑚𝑙 = +3; 𝑚𝑠 = + 1Τ2
b. 𝑛 = 3; 𝑙 = 2; 𝑚𝑙 = 0; 𝑚𝑠 = + 1Τ2
c. 𝑛 = 3; 𝑙 = 3; 𝑚𝑙 = 0; 𝑚𝑠 = + 1Τ2
d. 𝑛 = 3; 𝑙 = 3; 𝑚𝑙 = +3; 𝑚𝑠 = + 1Τ2
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
18. Which among the sets of quantum numbers is
valid and an electron can be found?
a. 𝑛 = 3; 𝑙 = 2; 𝑚𝑙 = +3; 𝑚𝑠 = + 1Τ2
b. 𝑛 = 3; 𝑙 = 2; 𝑚𝑙 = 0; 𝑚𝑠 = + 1Τ2
c. 𝑛 = 3; 𝑙 = 3; 𝑚𝑙 = 0; 𝑚𝑠 = + 1Τ2
d. 𝑛 = 3; 𝑙 = 3; 𝑚𝑙 = +3; 𝑚𝑠 = + 1Τ2
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
19. What is the molecular geometry of 𝑋𝑒𝐹4
a. Tetrahedral
b.Square planar
c. Sawhorse
d.Bent
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
19. What is the molecular geometry of 𝑋𝑒𝐹4
a. Tetrahedral
b.Square planar
c. Sawhorse
d.Bent
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
20. It is a molecule that contains positive and
negative poles. They tend to orient themselves in the
presence of an electric field.
a. Paramagnetic
b.Diamagnetic
c. Polar
d.Non-polar
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
20. It is a molecule that contains positive and
negative poles. They tend to orient themselves in the
presence of an electric field.
a. Paramagnetic
b.Diamagnetic
c. Polar
d.Non-polar
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
21. Determine which type of intermolecular forces of
attraction is most prevalent between 𝐶𝐶𝑙4 and
𝐼2 compounds.
a. London Dispersion Forces
b.Dipole-Dipole Forces
c. Hydrogen Bonding
d.Ion-Dipole Forces
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
21. Determine which type of intermolecular forces of
attraction is most prevalent between 𝐶𝐶𝑙4 and
𝐼2 compounds.
a. London Dispersion Forces
b.Dipole-Dipole Forces
c. Hydrogen Bonding
d.Ion-Dipole Forces
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
22. What kind of solution forms when gasoline
evaporates in air?
a. Gas in gas solution
b.Gas in liquid solution
c. Liquid in gas solution
d.Liquid in liquid solution
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
22. What kind of solution forms when gasoline
evaporates in air?
a. Gas in gas solution
b.Gas in liquid solution
c. Liquid in gas solution
d.Liquid in liquid solution
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
23. Of the following, which will increase the
solubility of gas in water?
a. Increasing the temperature
b.Increasing the pressure
c. Increasing the volume
d.Increasing the density
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
23. Of the following, which will increase the
solubility of gas in water?
a. Increasing the temperature
b.Increasing the pressure
c. Increasing the volume
d.Increasing the density
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
24. An 80 PROOF alcohol contains _____.
a. 40 mL alcohol in 100 mL water
b.40 mL alcohol in 60 mL water
c. 60 mL alcohol in 100 mL water
d.60 mL alcohol in 40 mL water
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
24. An 80 PROOF alcohol contains _____.
a. 40 mL alcohol in 100 mL water
b.40 mL alcohol in 60 mL water
c. 60 mL alcohol in 100 mL water
d.60 mL alcohol in 40 mL water
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
25. What volume is required of 2.0 M NaOH to make
150 mL of 1.0 M NaOH?
a. 25 mL
b.50 mL
c. 75 mL
d.200 mL
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
25. What volume is required of 2.0 M NaOH to make
150 mL of 1.0 M NaOH?
a. 25 mL
b.50 mL
c. 75 mL
d.200 mL
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
26. It is the phenomenon in which more than one
compound has the same chemical formula but
different chemical structures.
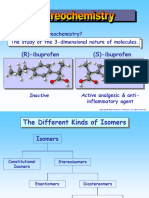
a. Isomerism
b.Enantiomers
c. Stereochemistry
d.Stereoisomerism
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
26. It is the phenomenon in which more than one
compound has the same chemical formula but
different chemical structures.
a. Isomerism
b.Enantiomers
c. Stereochemistry
d.Stereoisomerism
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
27. It refers to a carbon atom that is attached to four
different substituents, that are placed at the corner
of a tetrahedron.
a. Centers
b.Chiral
c. Wedged
d.Achiral
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
27. It refers to a carbon atom that is attached to four
different substituents, that are placed at the corner
of a tetrahedron.
a. Centers
b.Chiral
c. Wedged
d.Achiral
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
28. In the molecule (2S)-2,3-dihydroxypropanal, the
arrangement of the substituents, in terms of priority,
of the chiral carbon is __________.
a. Clockwise
b.Counterclockwise
c. Upwards
d.Downwards
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
28. In the molecule (2S)-2,3-dihydroxypropanal, the
arrangement of the substituents, in terms of priority,
of the chiral carbon is __________.
a. Clockwise
b.Counterclockwise
c. Upwards
d.Downwards
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
29. It is a type of organic reaction in which an atom
or group of atoms in a molecule change position to a
more stable location.
a. Addition
b.Elimination
c. Substitution
d.Rearrangements
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
29. It is a type of organic reaction in which an atom
or group of atoms in a molecule change position to a
more stable location.
a. Addition
b.Elimination
c. Substitution
d.Rearrangements
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
30. These are electron-deficient chemical species
which are typically neutral and/or positively-charged
and that they are being added or used to substitute
in a certain organic reaction.
a. Electrophiles
b.Nucleophiles
c. Photons
d.Quarks
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
30. These are electron-deficient chemical species
which are typically neutral and/or positively-charged
and that they are being added or used to substitute
in a certain organic reaction.
a. Electrophiles
b.Nucleophiles
c. Photons
d.Quarks
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
31. It is the carbon in which the amino group,
carboxylic group, and variable group were attached
to form amino acids.
a. -carbon
b.β-carbon
c. -glycosidic
d.β-glycosidic
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
31. It is the carbon in which the amino group,
carboxylic group, and variable group were attached
to form amino acids.
a. -carbon
b.β-carbon
c. -glycosidic
d.β-glycosidic
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
32. It is the carbon in which the amino group,
carboxylic group, and variable group were attached
to form amino acids.
a. Peptide bond
b.Glycosidic bond
c. Phosphodiester bond
d.Hydrogen bond
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
32. It is the carbon in which the amino group,
carboxylic group, and variable group were attached
to form amino acids.
a. Peptide bond
b.Glycosidic bond
c. Phosphodiester bond
d.Hydrogen bond
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
33. When balancing chemical equations, which of
the following cannot be changed?
a. Subscripts
b.Coefficients
c. Both subscripts and coefficients
d.Neither subscripts nor coefficients
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
33. When balancing chemical equations, which of
the following cannot be changed?
a. Subscripts
b.Coefficients
c. Both subscripts and coefficients
d.Neither subscripts nor coefficients
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
34. What is a limiting reactant in a chemical
reaction?
a. The reactant that is consumed first and limits
the extent of the reaction
b.The reactant with the highest molar mass
c. The reactant present in the highest quantity
d.The reactant that is always in excess
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
34. What is a limiting reactant in a chemical
reaction?
a. The reactant that is consumed first and limits
the extent of the reaction
b.The reactant with the highest molar mass
c. The reactant present in the highest quantity
d.The reactant that is always in excess
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
35. Which of the following statements is true about
oxidation and reduction?
a. Oxidation involves the loss of electrons, and reduction involves
the gain of electrons.
b. Oxidation involves the gain of electrons, and reduction involves
the loss of electrons.
c. Oxidation involves the gain of electrons, and reduction involves
the loss of electrons.
d. Oxidation involves the loss of protons, and reduction involves
the gain of protons.
Saint Michael College of Caraga
CHEMISTRY LET REVIEW
35. Which of the following statements is true about
oxidation and reduction?
a. Oxidation involves the loss of electrons, and reduction involves
the gain of electrons.
b. Oxidation involves the gain of electrons, and reduction involves
the loss of electrons.
c. Oxidation involves the gain of protons, and reduction involves
the loss of protons.
d. Oxidation involves the loss of protons, and reduction involves
the gain of protons.
Saint Michael College of Caraga
You might also like
- Organic Chemistry NotesDocument45 pagesOrganic Chemistry NotesJasmine Sloan100% (1)
- Stereochemistry NotesDocument86 pagesStereochemistry NotesDr. Krishna Swamy G100% (1)
- CBSE Class 11 Chemistry - RevisionDocument3 pagesCBSE Class 11 Chemistry - RevisionPrasad YarraNo ratings yet
- Chemistry NMAT Questionnaire by NMAT Study BuddyDocument10 pagesChemistry NMAT Questionnaire by NMAT Study BuddyJohn Rhel DenqueNo ratings yet
- CH 8 Test BankDocument14 pagesCH 8 Test BankVanessa James100% (2)
- Chemistry Practice Test With Answer For Physical Science MajorDocument6 pagesChemistry Practice Test With Answer For Physical Science MajorJesmar Quirino TutingNo ratings yet
- Answer Key - Chemistry - Diagnostic ExamDocument2 pagesAnswer Key - Chemistry - Diagnostic ExamNiño Edrianne Nimo100% (2)
- StereochemistryDocument53 pagesStereochemistryDeshDeepak AbhayPratap SinghChauhan100% (1)
- AnswersDocument14 pagesAnswersNasser Gemina PantaoNo ratings yet
- StereochemistryDocument78 pagesStereochemistryApurba Sarker Apu100% (10)
- Physical and Chemical PrinciplesDocument16 pagesPhysical and Chemical PrinciplesJD6 AgarbNo ratings yet
- Chemistry QuestionsDocument71 pagesChemistry QuestionsNnaer Ortiz NasupmilacNo ratings yet
- Preboard Exam Day1 SET ADocument12 pagesPreboard Exam Day1 SET AJeanne Cortez0% (1)
- ExChEL Group Study Session 13 - Day 1 ExaminationDocument15 pagesExChEL Group Study Session 13 - Day 1 ExaminationRochelle Louise SampagaNo ratings yet
- Basic Education Department (SHS) : Qualifying Examination - ChemistryDocument3 pagesBasic Education Department (SHS) : Qualifying Examination - Chemistryismael jaafarNo ratings yet
- General ChemistryDocument4 pagesGeneral ChemistryKrizzia Anne ShengNo ratings yet
- Midyear Assessment General Chemistry 1Document7 pagesMidyear Assessment General Chemistry 1Jabeguero Marvelyn JessicaNo ratings yet
- My FilesDocument18 pagesMy Filesjake dionisioNo ratings yet
- General-Inorganic-Chemistry-Review BY ENGR JANMELLDocument62 pagesGeneral-Inorganic-Chemistry-Review BY ENGR JANMELLMichael Vincent Mirafuentes100% (1)
- Orca Share Media1541759628568 PDFDocument12 pagesOrca Share Media1541759628568 PDFJuneNeilBalacuitNo ratings yet
- MCQ in General Chemistry Part 13 - ECE Board ExamDocument10 pagesMCQ in General Chemistry Part 13 - ECE Board ExamDominic Nicole ManuelNo ratings yet
- ReviewerDocument6 pagesReviewerMariAntonetteChangNo ratings yet
- SCH3U - Practice ExamDocument9 pagesSCH3U - Practice ExamWaqas AhmadNo ratings yet
- The Principles of Heterocyclic ChemistryFrom EverandThe Principles of Heterocyclic ChemistryRating: 3 out of 5 stars3/5 (2)
- Chemistry Let ReviewDocument77 pagesChemistry Let ReviewCawen TaponNo ratings yet
- Chem15 ProbSet1Document4 pagesChem15 ProbSet1Eiza May BaLaguerNo ratings yet
- 11 Chemistry Sample Paper 01Document16 pages11 Chemistry Sample Paper 01loduuNo ratings yet
- Science ReviewDocument85 pagesScience ReviewRussell VenturaNo ratings yet
- PNCO Questions 1Document38 pagesPNCO Questions 1Garcia RaphNo ratings yet
- Mastery Test in Physical Science - Gr.11Document3 pagesMastery Test in Physical Science - Gr.11kert mendozaNo ratings yet
- Second Term ExaminationDocument5 pagesSecond Term ExaminationAriyo olawaleNo ratings yet
- Chemistry Entry Test 2017 2018Document7 pagesChemistry Entry Test 2017 2018Collen Tinashe MakoniNo ratings yet
- Tejas Institute Physics First MCQ TestDocument3 pagesTejas Institute Physics First MCQ TestKB ChoudhuryNo ratings yet
- Directions: This Examination Contains A Total of 80 Multiple ChoiceDocument12 pagesDirections: This Examination Contains A Total of 80 Multiple ChoiceLemi NegesoNo ratings yet
- 1127 Practice FinalDocument8 pages1127 Practice FinalRyan GrijalvaNo ratings yet
- CHEMISTRY Model ExamDocument7 pagesCHEMISTRY Model ExamBereket AsefaNo ratings yet
- Mock Board Examination: Physical and Chemical Principles: Saint Louis University - Sea - Chemical EngineringDocument6 pagesMock Board Examination: Physical and Chemical Principles: Saint Louis University - Sea - Chemical EngineringZZROTNo ratings yet
- WSFSC112 Mock ExamDocument8 pagesWSFSC112 Mock ExamRaphaelNo ratings yet
- Chem 16 Finals SamplexDocument3 pagesChem 16 Finals SamplexKayeNo ratings yet
- Chemistry IE Review2017Document4 pagesChemistry IE Review2017Rugi Vicente RubiNo ratings yet
- CHEMDocument5 pagesCHEMScam CheckNo ratings yet
- NAT Mock BIO CHEM PHYSICSDocument8 pagesNAT Mock BIO CHEM PHYSICSRafael PresadoNo ratings yet
- NMAT Chemistry Practice Questions Set 3Document9 pagesNMAT Chemistry Practice Questions Set 3Nurshayma JalilNo ratings yet
- Chemistry (Drill 1)Document4 pagesChemistry (Drill 1)Ellie PamintuanNo ratings yet
- RVM Nat Dry Run - Science IIIDocument7 pagesRVM Nat Dry Run - Science IIITidal SurgesNo ratings yet
- Phy ChemDocument14 pagesPhy ChemBabylyn AustriaNo ratings yet
- Exam 1 For CorreDocument3 pagesExam 1 For Correcamille lei CalderonNo ratings yet
- CHEM Chemistry in Your World 2nd Edition by Hogg ISBN 113396298X Test BankDocument11 pagesCHEM Chemistry in Your World 2nd Edition by Hogg ISBN 113396298X Test Bankstephanie100% (20)
- Test Bank For Chem 2 Chemistry in Your World 2Nd Edition by Hogg Isbn 113396298X 9781133962984 Full Chapter PDFDocument17 pagesTest Bank For Chem 2 Chemistry in Your World 2Nd Edition by Hogg Isbn 113396298X 9781133962984 Full Chapter PDFhenry.smith811100% (11)
- PreboardELEMGeneral Education PreboardDocument6 pagesPreboardELEMGeneral Education PreboardJezha Mae Vertudazo0% (1)
- 2 1 IQC QuestionsDocument10 pages2 1 IQC QuestionsJazyl TanNo ratings yet
- Final QuestionsDocument19 pagesFinal QuestionsyanyanNo ratings yet
- Sample Question Paper M - 1Document12 pagesSample Question Paper M - 1avinash solankiNo ratings yet
- Midterm Chem.Document3 pagesMidterm Chem.Joue sarsonasNo ratings yet
- gd2 PMDocument14 pagesgd2 PMKevin DacreNo ratings yet
- SummativeTest-Q2 Gr. 9Document5 pagesSummativeTest-Q2 Gr. 9Chee MaRieNo ratings yet
- Set ADocument6 pagesSet AJabeguero Marvelyn JessicaNo ratings yet
- Unit 1 Class 9Document2 pagesUnit 1 Class 9White RoseNo ratings yet
- Top Univ - Soal Latihan Kimia 01 PDFDocument7 pagesTop Univ - Soal Latihan Kimia 01 PDFDarma YogaNo ratings yet
- CHM-2045 Exam 1 Sample QuestionsDocument7 pagesCHM-2045 Exam 1 Sample QuestionsFrankNo ratings yet
- SHREE POKHARIYA SECONDARY SCHOOL Class 11 Tech.Document2 pagesSHREE POKHARIYA SECONDARY SCHOOL Class 11 Tech.pakheyyyNo ratings yet
- 10 Science Pre Board 1 8 JANDocument5 pages10 Science Pre Board 1 8 JANprejan rajaNo ratings yet
- Chemistry ModelDocument11 pagesChemistry Modelabdi belina100% (1)
- Ebook Smith and Aitkenheads Textbook of Anaesthesia 7Th Edition PDF Full Chapter PDFDocument67 pagesEbook Smith and Aitkenheads Textbook of Anaesthesia 7Th Edition PDF Full Chapter PDFelaine.kern334100% (23)
- Topic 4. Stereochemistry (Chapter 5) : ObjectivesDocument19 pagesTopic 4. Stereochemistry (Chapter 5) : ObjectivesMamatha K NNo ratings yet
- Haloalkanes and HaloarenesDocument19 pagesHaloalkanes and HaloarenesHemanth GowdaNo ratings yet
- Introduction To AlkanesDocument29 pagesIntroduction To AlkanesJasminSutkovicNo ratings yet
- F324 All QuestionsDocument95 pagesF324 All QuestionsIntesar NurNo ratings yet
- Stereochemistry Lecture 1Document22 pagesStereochemistry Lecture 1Divyakumar PatelNo ratings yet
- Stereokimia: Fasilitator: Dr. Marcellino RudyantoDocument33 pagesStereokimia: Fasilitator: Dr. Marcellino RudyantoAqielNo ratings yet
- CH 5. StereochemistryDocument36 pagesCH 5. StereochemistryAhmed ZakyNo ratings yet
- Stre Okimi ADocument73 pagesStre Okimi A2008Muhammad Rifaldi DirgantaraNo ratings yet
- Class Notes Mondal IsomerismDocument49 pagesClass Notes Mondal IsomerismDeepanshu 1459No ratings yet
- Isomerism ReviewDocument7 pagesIsomerism Reviewayesha sheikhNo ratings yet
- Lab Report Template - 230507 - 092259Document49 pagesLab Report Template - 230507 - 092259WAN AZALEEYA BINTI WAN AZANI / UPMNo ratings yet
- ChemistryDocument48 pagesChemistryloretta00No ratings yet
- Organic Chemistry 9Th Edition Mcmurry Test Bank Full Chapter PDFDocument34 pagesOrganic Chemistry 9Th Edition Mcmurry Test Bank Full Chapter PDFmaria.topolosky417100% (10)
- Isomerism KEC 077 Lecture IV BCE A 079-02-17 PST.Document26 pagesIsomerism KEC 077 Lecture IV BCE A 079-02-17 PST.bsarad115No ratings yet
- R, S - NomenclatureDocument19 pagesR, S - Nomenclatureimsc2022uohNo ratings yet
- MC Murry 9Document61 pagesMC Murry 9JoseNo ratings yet
- Che 220: Stereochemistry: Chirality & EnantiomersDocument34 pagesChe 220: Stereochemistry: Chirality & EnantiomersShadrack MaduluNo ratings yet
- Biochemistry Prelim NotesDocument7 pagesBiochemistry Prelim NotesCeline Ricci AbrahamNo ratings yet
- L5 Isomerism 3Document16 pagesL5 Isomerism 3Cheng FuNo ratings yet
- Questions and Answers - Optical IsomerismDocument15 pagesQuestions and Answers - Optical IsomerismMurali GNo ratings yet
- Stereo CenterDocument2 pagesStereo CenterBrinzei LucianNo ratings yet
- bcm.04 MoleculesDocument21 pagesbcm.04 MoleculeslauderNo ratings yet
- Curso de Quimica IDocument233 pagesCurso de Quimica IEmanuelRomeroGNo ratings yet
- StereochemistryDocument108 pagesStereochemistryAllan DNo ratings yet
- Chapter 5 ProblemsDocument7 pagesChapter 5 Problemsdicoz diphaNo ratings yet