Professional Documents
Culture Documents
The Equilibrium Constant
Uploaded by
usulasia7770 ratings0% found this document useful (0 votes)
4 views2 pagesThe equilibrium constant (K) is a numerical value that expresses the ratio of product to reactant concentrations at equilibrium. K depends on temperature but has no units. A K > 1 favors products and a K < 1 favors reactants. Kc uses concentrations and Kp uses partial pressures. Kw represents the ion product of water. K is determined experimentally or from thermodynamic data and is used for equilibrium calculations in chemistry, engineering, and biochemistry, though it provides no kinetic information.
Original Description:
Original Title
The equilibrium constant
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe equilibrium constant (K) is a numerical value that expresses the ratio of product to reactant concentrations at equilibrium. K depends on temperature but has no units. A K > 1 favors products and a K < 1 favors reactants. Kc uses concentrations and Kp uses partial pressures. Kw represents the ion product of water. K is determined experimentally or from thermodynamic data and is used for equilibrium calculations in chemistry, engineering, and biochemistry, though it provides no kinetic information.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
4 views2 pagesThe Equilibrium Constant
Uploaded by
usulasia777The equilibrium constant (K) is a numerical value that expresses the ratio of product to reactant concentrations at equilibrium. K depends on temperature but has no units. A K > 1 favors products and a K < 1 favors reactants. Kc uses concentrations and Kp uses partial pressures. Kw represents the ion product of water. K is determined experimentally or from thermodynamic data and is used for equilibrium calculations in chemistry, engineering, and biochemistry, though it provides no kinetic information.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
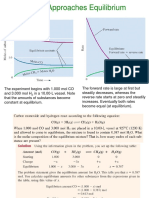
The equilibrium constant
1. Definition:
● The equilibrium constant (K) is a numerical value that expresses the ratio of
the concentrations of products to the concentrations of reactants at
equilibrium, with each concentration raised to the power of its stoichiometric
coefficient. For a general reaction:
● aA+bB⇌cC+dD
The equilibrium constant, K, is given by:
● K=[C]^c [D]^d/[A]^a [B]^b
2. Characteristics of Equilibrium Constants:
● Temperature Dependence: The value of the equilibrium constant (K) is
temperature-dependent. Changes in temperature can alter the equilibrium
constant for a reaction, leading to shifts in the equilibrium position.
● Dimensionless: Equilibrium constants are dimensionless quantities since the
concentrations of reactants and products are expressed in moles per liter (M).
Therefore, the equilibrium constant has no units.
● Magnitude of K: The magnitude of the equilibrium constant provides
information about the position of equilibrium. A large value of K (>1) indicates
that the reaction favors the formation of products at equilibrium, while a small
value of K (<1) indicates that the reaction favors the formation of reactants at
equilibrium.
3. Types of Equilibrium Constants:
● Kc: The equilibrium constant expressed in terms of concentrations (in moles
per liter) of reactants and products.
● Kp: The equilibrium constant expressed in terms of partial pressures (in
atmospheres) of reactants and products for gaseous reactions.
● Kw: The ion product constant for water, which represents the equilibrium
constant for the autoionization of water into hydronium ions (H₃O⁺) and
hydroxide ions (OH⁻) in aqueous solutions.
4. Calculation of Equilibrium Constants:
● Equilibrium constants are determined experimentally by measuring the
concentrations of reactants and products at equilibrium and using these
values to calculate the equilibrium constant using the appropriate expression
(Kc or Kp).
● Alternatively, equilibrium constants can be calculated using thermodynamic
data such as standard free energy changes (∆G°) and the relationship
between equilibrium constants and thermodynamic parameters.
5. Applications of Equilibrium Constants:
● Equilibrium constants are used extensively in chemical equilibrium
calculations to predict the direction in which a reaction will proceed and to
determine the concentrations of reactants and products at equilibrium.
● They are crucial in various fields of chemistry, including chemical engineering,
environmental science, and biochemistry, for designing and optimizing
chemical processes, analyzing environmental systems, and understanding
biochemical reactions.
6. Limitations of Equilibrium Constants:
● Equilibrium constants provide information about the position of equilibrium
but do not provide insights into the rate at which equilibrium is achieved or the
kinetics of the reaction.
● They assume ideal conditions, such as perfect mixing, constant temperature,
and a closed system. Deviations from these conditions can affect the
accuracy of predictions based on equilibrium constants.
You might also like
- The Equilibrium LawDocument2 pagesThe Equilibrium Lawusulasia777No ratings yet
- Chemical EquilibriumDocument46 pagesChemical EquilibriumMary Rose AguilaNo ratings yet
- Chemical EquilibriumDocument4 pagesChemical EquilibriumDraver ZemaNo ratings yet
- Chapter 5 Chemical EquilibriumDocument36 pagesChapter 5 Chemical Equilibriumfit3akmalNo ratings yet
- Class 11 Chemistry Revision Notes EquilibriumDocument14 pagesClass 11 Chemistry Revision Notes EquilibriumSwastika DasNo ratings yet
- Chemical Equilibrium - Types, Principles and Laws of EquilibriaDocument13 pagesChemical Equilibrium - Types, Principles and Laws of EquilibriaRafael TayoNo ratings yet
- Slide Chapter 2 Chemical Equlibrium ASC0305 MJHDocument72 pagesSlide Chapter 2 Chemical Equlibrium ASC0305 MJHEza GuinNo ratings yet
- Chapter 4 Part 1Document18 pagesChapter 4 Part 1gbygbybNo ratings yet
- Chemical Equilibria-1Document50 pagesChemical Equilibria-1fmukuka12No ratings yet
- TSFX Chem Tips PDFDocument19 pagesTSFX Chem Tips PDFIra HenryNo ratings yet
- Study of Kinetics and Thermodynamics of A ReactionDocument19 pagesStudy of Kinetics and Thermodynamics of A ReactionAnonymous eDyMaC6CNo ratings yet
- 2 Chemical Equilibrium - FDocument94 pages2 Chemical Equilibrium - FSelena Dela CruzNo ratings yet
- Hilts 105Document277 pagesHilts 105Wesley CheungNo ratings yet
- Chemical EquilibriaDocument50 pagesChemical EquilibriaDominic ReignsNo ratings yet
- Chemical Equilibrium NotesDocument4 pagesChemical Equilibrium NotesHaile CordaNo ratings yet
- Chemistry Unit 4 Part 3 ReallyacademicsDocument35 pagesChemistry Unit 4 Part 3 ReallyacademicsWill AndyNo ratings yet
- EQUILUBRIUMDocument31 pagesEQUILUBRIUMcdsingh74No ratings yet
- 7.1 EquilibriumDocument43 pages7.1 Equilibriummotor impulseNo ratings yet
- Chemical Equlibrium (Autosaved)Document21 pagesChemical Equlibrium (Autosaved)iqbal-cheNo ratings yet
- Equilibrium ReactionDocument2 pagesEquilibrium ReactionAlif Nuzulul HidayatNo ratings yet
- Equilibrium Class XiDocument47 pagesEquilibrium Class XilololberuhlololNo ratings yet
- Unit 7: Equilibrium: Introduction To Equilibrium and Reversible ReactionsDocument5 pagesUnit 7: Equilibrium: Introduction To Equilibrium and Reversible ReactionsRylee LippenholzNo ratings yet
- 06 S and P Block Elements Que. Final E 2Document10 pages06 S and P Block Elements Que. Final E 2gnkstarNo ratings yet
- Chemistry HandoutDocument15 pagesChemistry Handoutprasanta_bbsrNo ratings yet
- ws14 1Document6 pagesws14 1Diana Jean Alo-adNo ratings yet
- ws14 1Document6 pagesws14 1Evilasio CostaNo ratings yet
- ws14 1Document6 pagesws14 1Evilasio CostaNo ratings yet
- Chapter 7 EquilibriumDocument5 pagesChapter 7 Equilibriumapi-392847673No ratings yet
- Lesson 2 - 7 2Document10 pagesLesson 2 - 7 2api-281535910No ratings yet
- Lecture-Unit 9 Chemical EquilibriumDocument13 pagesLecture-Unit 9 Chemical EquilibriumKemoy FrancisNo ratings yet
- CHAP.4 Thermo-1Document71 pagesCHAP.4 Thermo-1btesfaye168No ratings yet
- Chapter17 (Chemical Equilibria)Document61 pagesChapter17 (Chemical Equilibria)joshuaNo ratings yet
- Equilibrium Constant and Factors Affecting EquilibriumDocument24 pagesEquilibrium Constant and Factors Affecting EquilibriumRicardo Jr. UyNo ratings yet
- Kimdas Equilibrium Eng PDFDocument76 pagesKimdas Equilibrium Eng PDFO2TROPI RamadhanNo ratings yet
- EquilibriumDocument10 pagesEquilibriumKainshk GuptaNo ratings yet
- CONCEPTS OF CHEMICAL EQUILIBRIUM Chapter 6Document6 pagesCONCEPTS OF CHEMICAL EQUILIBRIUM Chapter 6juliannekirstenrNo ratings yet
- Q4 WEEK 2 Gen Chem 2 Worksheet 11 CHEMICAL EQUILIBRIUMDocument9 pagesQ4 WEEK 2 Gen Chem 2 Worksheet 11 CHEMICAL EQUILIBRIUMMarielle TibayNo ratings yet
- Law of Mass ActionDocument17 pagesLaw of Mass ActionKoomal KafaitNo ratings yet
- Equilibrium NotesDocument14 pagesEquilibrium NotessusrudhansNo ratings yet
- Edexcel IAL Chemistry A-Level: Topic 13: Chemical EquilibriaDocument6 pagesEdexcel IAL Chemistry A-Level: Topic 13: Chemical EquilibriaMer CyNo ratings yet
- Chemical Equilibrium-UAPDocument26 pagesChemical Equilibrium-UAPMd. Mujahid HasanNo ratings yet
- CHM 111 General Chemistry I: Department of Chemistry University of Medical Sciences Ondo, Ondo State, NigeriaDocument16 pagesCHM 111 General Chemistry I: Department of Chemistry University of Medical Sciences Ondo, Ondo State, Nigeriaomelle AbubakarNo ratings yet
- 14 EquilibriumDocument6 pages14 EquilibriumAgam HanasichulaNo ratings yet
- Chapter 13chemical EquilibriumDocument5 pagesChapter 13chemical EquilibriumKevin HuangNo ratings yet
- EquilibriumDocument5 pagesEquilibriumnanaNo ratings yet
- Chemical EqulibriumDocument23 pagesChemical Equlibriumiqbal-cheNo ratings yet
- IB Chemistry 7.1 NotesDocument4 pagesIB Chemistry 7.1 NotesRadhika12345No ratings yet
- Chapter14 Moore ChemicLEquilibriumDocument18 pagesChapter14 Moore ChemicLEquilibriumRioneli GhaudensonNo ratings yet
- 13 General EquilibriumDocument11 pages13 General Equilibriumapi-234034801No ratings yet
- Chemical Equilibrium NotesDocument3 pagesChemical Equilibrium NotesusmanzubairNo ratings yet
- Chemical Equilibrium 01Document13 pagesChemical Equilibrium 01Robin TimkangNo ratings yet
- 7.1 EquilibriumDocument42 pages7.1 EquilibriumMirai KurooNo ratings yet
- Chapter 8 EquilibriumDocument99 pagesChapter 8 EquilibriumSimple qaseemNo ratings yet
- Equilibrium - NotesDocument10 pagesEquilibrium - NotesAliza IsmailNo ratings yet
- Chemical EquilibriumDocument30 pagesChemical EquilibriumSachin KumarNo ratings yet
- Chapter 6 Chemical Reaction EquilbrumDocument22 pagesChapter 6 Chemical Reaction EquilbrummohammedNo ratings yet
- Chapter 3 Chemical EquilibriumDocument35 pagesChapter 3 Chemical EquilibriumSuraj BhattaraiNo ratings yet
- Practice Makes Perfect in Chemistry: The Physical Behavior of MatterFrom EverandPractice Makes Perfect in Chemistry: The Physical Behavior of MatterRating: 5 out of 5 stars5/5 (1)
- Basic Electrical Theory Study Sheet (v3.1)Document1 pageBasic Electrical Theory Study Sheet (v3.1)Rick GouldNo ratings yet
- Machine DesignDocument10 pagesMachine DesignKIMBERLY MIRANDANo ratings yet
- Dinamica - Presentacion 2Document24 pagesDinamica - Presentacion 2Jhonny CarbonóNo ratings yet
- Transient Stability Analysis of Grid Wind Power Plant Pettikkattil Radhakrishnan-Jaikumar-thesis-2014Document76 pagesTransient Stability Analysis of Grid Wind Power Plant Pettikkattil Radhakrishnan-Jaikumar-thesis-2014Armando MartinezNo ratings yet
- Oldroyd B FluidDocument8 pagesOldroyd B FluidAslıhan örümNo ratings yet
- MOSFET Devices: Gu-Yeon Wei Division of Engineering and Applied Sciences Harvard University Guyeon@eecs - Harvard.eduDocument27 pagesMOSFET Devices: Gu-Yeon Wei Division of Engineering and Applied Sciences Harvard University Guyeon@eecs - Harvard.eduDr-Ahmed ElkoranyNo ratings yet
- Complete Physics Notes First YearDocument215 pagesComplete Physics Notes First YearJaved Ahmed Chandio100% (2)
- AssgnmntDocument10 pagesAssgnmntAyan SahaNo ratings yet
- Screeds: A Wirtgen Group CompanyDocument29 pagesScreeds: A Wirtgen Group CompanyMichael Forrest100% (1)
- Vd10-11-Axian Piros Winder 48-1000t-6 7.2 KW - UkDocument2 pagesVd10-11-Axian Piros Winder 48-1000t-6 7.2 KW - UkConstantin294No ratings yet
- MII: Vehicle Dynamics: Ashok Jhunjhunwala and Prabhjot Kaur, IIT MadrasDocument36 pagesMII: Vehicle Dynamics: Ashok Jhunjhunwala and Prabhjot Kaur, IIT MadrasKarthik rNo ratings yet
- 16.SIE.4AM - 4AT... Transformers Technical Data - EnglishDocument8 pages16.SIE.4AM - 4AT... Transformers Technical Data - Englishsaat plcNo ratings yet
- Force Equals Mass Times AccelerationDocument1 pageForce Equals Mass Times AccelerationDen EsicNo ratings yet
- Flight PlanningDocument6 pagesFlight PlanningShruti PrakasenNo ratings yet
- Carta Sicrometrica A Nivel Del MarDocument1 pageCarta Sicrometrica A Nivel Del MarEstebanCórdobaNo ratings yet
- The Wave Equation: & Speed of WavesDocument21 pagesThe Wave Equation: & Speed of WavesCaryl Jane Escatin BeatoNo ratings yet
- Diesel Generator Sets: Standard FeaturesDocument5 pagesDiesel Generator Sets: Standard FeaturesHany FataNo ratings yet
- The IEC Aerodynamic Valve Noise - OK OK OK TEORIADocument4 pagesThe IEC Aerodynamic Valve Noise - OK OK OK TEORIAJOSE MARTIN MORA RIVEROSNo ratings yet
- FLUENT - Flow Over An Airfoil - Simulation - ConfluenceDocument24 pagesFLUENT - Flow Over An Airfoil - Simulation - ConfluenceFabrice Nebesse100% (2)
- Brosura TPDocument6 pagesBrosura TPgreenpeaceromNo ratings yet
- Infineon XDPS2201 DataSheet v01 - 10 ENDocument75 pagesInfineon XDPS2201 DataSheet v01 - 10 EN偉誠曾No ratings yet
- Design Solutions For DC Bias of Multilayer Ceramic Capactiors (MLCCS)Document3 pagesDesign Solutions For DC Bias of Multilayer Ceramic Capactiors (MLCCS)Nikola DulgiarovNo ratings yet
- ANTAI Calculation 40m Per Second ASCE 7-10Document13 pagesANTAI Calculation 40m Per Second ASCE 7-10Yên BìnhNo ratings yet
- Project ReportDocument73 pagesProject Reportrajat123sharma60% (15)
- Fundamentals of EMC DesignDocument12 pagesFundamentals of EMC DesignkwastekNo ratings yet
- Performance Analysis of Fast Charging Stations For G2V and V2G Micro-Grid SystemsDocument7 pagesPerformance Analysis of Fast Charging Stations For G2V and V2G Micro-Grid SystemsInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Motion Graphs Displacement-Time GraphsDocument11 pagesMotion Graphs Displacement-Time GraphsprimalNo ratings yet
- CH 14Document59 pagesCH 14Muhammad Tayyab Madni100% (1)
- FCU ChecklistDocument11 pagesFCU ChecklistAnsari1918No ratings yet
- LRS Operation ManualDocument21 pagesLRS Operation Manual2003vinayNo ratings yet