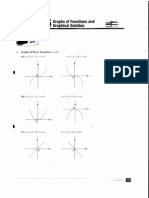
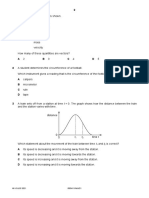
Professional Documents
Culture Documents
The Equilibrium Law
Uploaded by
usulasia7770 ratings0% found this document useful (0 votes)
4 views2 pagesThe equilibrium law states that for a chemical reaction at equilibrium, the ratio of the product of the concentrations of the products raised to their stoichiometric coefficients to the product of the concentrations of the reactants raised to their stoichiometric coefficients is a constant known as the equilibrium constant (Kc). The value of Kc provides information about the position of equilibrium and favors the formation of either products or reactants. The equilibrium constant is applied in various areas of chemistry to predict reaction outcomes and calculate concentrations at equilibrium.
Original Description:
Original Title
The equilibrium law
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe equilibrium law states that for a chemical reaction at equilibrium, the ratio of the product of the concentrations of the products raised to their stoichiometric coefficients to the product of the concentrations of the reactants raised to their stoichiometric coefficients is a constant known as the equilibrium constant (Kc). The value of Kc provides information about the position of equilibrium and favors the formation of either products or reactants. The equilibrium constant is applied in various areas of chemistry to predict reaction outcomes and calculate concentrations at equilibrium.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
4 views2 pagesThe Equilibrium Law
Uploaded by
usulasia777The equilibrium law states that for a chemical reaction at equilibrium, the ratio of the product of the concentrations of the products raised to their stoichiometric coefficients to the product of the concentrations of the reactants raised to their stoichiometric coefficients is a constant known as the equilibrium constant (Kc). The value of Kc provides information about the position of equilibrium and favors the formation of either products or reactants. The equilibrium constant is applied in various areas of chemistry to predict reaction outcomes and calculate concentrations at equilibrium.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
The equilibrium law
1. Definition:
● The equilibrium law states that for a chemical reaction at equilibrium, the ratio
of the product of the concentrations of the products raised to their
stoichiometric coefficients to the product of the concentrations of the
reactants raised to their stoichiometric coefficients is a constant at a given
temperature. This constant is known as the equilibrium constant (Kc).
2. Equilibrium Constant (Kc):
● The equilibrium constant (Kc) is a quantitative measure of the extent to which
a chemical reaction proceeds to form products at equilibrium, based on the
concentrations of reactants and products. It is expressed as the ratio of the
concentrations of the products to the concentrations of the reactants, each
raised to the power of their stoichiometric coefficients.
● The general expression for the equilibrium constant for a reaction:
● For the reaction: aA + bB ⇌ cC + dD
3. Characteristics of Equilibrium Constants:
● Dependence on Temperature: The value of the equilibrium constant (Kc) is
temperature-dependent. Changes in temperature can alter the equilibrium
constant for a reaction, leading to shifts in the equilibrium position.
● Dimensionless: Equilibrium constants are dimensionless quantities since the
concentrations of reactants and products are expressed in moles per liter (M).
Therefore, the equilibrium constant has no units.
● Magnitude of Kc: The magnitude of the equilibrium constant provides
information about the position of equilibrium. A large value of Kc (>1)
indicates that the reaction favors the formation of products at equilibrium,
while a small value of Kc (<1) indicates that the reaction favors the formation
of reactants at equilibrium.
4. Application of the Equilibrium Law:
● The equilibrium law is applied extensively in various areas of chemistry,
including chemical kinetics, industrial processes, environmental chemistry,
and biochemical reactions.
● It allows chemists to predict the direction in which a reaction will proceed to
reach equilibrium under given conditions and to calculate the concentrations
of reactants and products at equilibrium.
5. Limitations of the Equilibrium Law:
● The equilibrium law assumes ideal conditions, such as perfect mixing,
constant temperature, and a closed system. Deviations from these conditions
can affect the accuracy of predictions based on equilibrium constants.
● It applies only to systems at equilibrium, where the rates of the forward and
reverse reactions are equal. Therefore, it does not provide information about
reaction kinetics or the time required to reach equilibrium.
You might also like
- The Equilibrium ConstantDocument2 pagesThe Equilibrium Constantusulasia777No ratings yet
- Chemical EquilibriumDocument4 pagesChemical EquilibriumDraver ZemaNo ratings yet
- Chemical EquilibriumDocument46 pagesChemical EquilibriumMary Rose AguilaNo ratings yet
- On Chemical Eqiulibrium For G-11Document44 pagesOn Chemical Eqiulibrium For G-11lenlucy13frNo ratings yet
- Chemistry HandoutDocument15 pagesChemistry Handoutprasanta_bbsrNo ratings yet
- Chapter 7 EquilibriumDocument5 pagesChapter 7 Equilibriumapi-392847673No ratings yet
- Lesson 2 - 7 2Document10 pagesLesson 2 - 7 2api-281535910No ratings yet
- CHM111 - Chemical Equilibrium NoteDocument22 pagesCHM111 - Chemical Equilibrium NoteolufemisongNo ratings yet
- Equilibrium NotesDocument14 pagesEquilibrium NotessusrudhansNo ratings yet
- Class 11 Chemistry Revision Notes EquilibriumDocument14 pagesClass 11 Chemistry Revision Notes EquilibriumSwastika DasNo ratings yet
- Reversible ReactionsDocument2 pagesReversible Reactionsusulasia777No ratings yet
- Chem NotesDocument40 pagesChem NotesFelicia LeNo ratings yet
- Chemical EquilibriumDocument8 pagesChemical EquilibriumDavid PetalcurinNo ratings yet
- IB Chemistry 7.1 NotesDocument4 pagesIB Chemistry 7.1 NotesRadhika12345No ratings yet
- TSFX Chem Tips PDFDocument19 pagesTSFX Chem Tips PDFIra HenryNo ratings yet
- Law of Mass ActionDocument17 pagesLaw of Mass ActionKoomal KafaitNo ratings yet
- State of EquilibriumDocument25 pagesState of EquilibriumBrent Nillas ZuñigaNo ratings yet
- Topic 7-17 NotesDocument76 pagesTopic 7-17 NotesHamzaNo ratings yet
- Chemical Kinetics Water Water Chemical Kinetics Water WaterDocument38 pagesChemical Kinetics Water Water Chemical Kinetics Water WateranaNo ratings yet
- Stoich JhemDocument1 pageStoich JhemTango Jhecee Meir, D.No ratings yet
- EQUILUBRIUMDocument31 pagesEQUILUBRIUMcdsingh74No ratings yet
- Chemical KineticsDocument53 pagesChemical KineticsEuann MagtibayNo ratings yet
- Equilibrium ReactionDocument2 pagesEquilibrium ReactionAlif Nuzulul HidayatNo ratings yet
- 7.1 EquilibriumDocument43 pages7.1 Equilibriummotor impulseNo ratings yet
- Cream and Green Illustrative Science Project PresentationDocument22 pagesCream and Green Illustrative Science Project Presentationralphvallespin8No ratings yet
- EQUILIBRIUMDocument1 pageEQUILIBRIUMMohammed IliasNo ratings yet
- Chemistry Notes PDFDocument7 pagesChemistry Notes PDFEngwa Clintine NdumbiNo ratings yet
- Chemical Equilibrium - Types, Principles and Laws of EquilibriaDocument13 pagesChemical Equilibrium - Types, Principles and Laws of EquilibriaRafael TayoNo ratings yet
- Chapter17 (Chemical Equilibria)Document61 pagesChapter17 (Chemical Equilibria)joshuaNo ratings yet
- CKRD-MS-02 (2020)Document133 pagesCKRD-MS-02 (2020)Shakoor MalikNo ratings yet
- Chapter 4 Part 1Document18 pagesChapter 4 Part 1gbygbybNo ratings yet
- Lesson 13 - Introduction To Equilibrium ConstantsDocument28 pagesLesson 13 - Introduction To Equilibrium ConstantsViper PotNo ratings yet
- EquilibriumDocument10 pagesEquilibriumKainshk GuptaNo ratings yet
- Principles of Chemical EquilibriumDocument17 pagesPrinciples of Chemical EquilibriumkaditasookdeoNo ratings yet
- Thermo 8e Chap 16 LectureDocument36 pagesThermo 8e Chap 16 LectureMutaz Al-MuhtasebNo ratings yet
- EquilibriumDocument4 pagesEquilibriumBikave JohnsonNo ratings yet
- Equilibrium ConstantDocument2 pagesEquilibrium ConstantSaad NaumanNo ratings yet
- Slide Chapter 2 Chemical Equlibrium ASC0305 MJHDocument72 pagesSlide Chapter 2 Chemical Equlibrium ASC0305 MJHEza GuinNo ratings yet
- Study of Kinetics and Thermodynamics of A ReactionDocument19 pagesStudy of Kinetics and Thermodynamics of A ReactionAnonymous eDyMaC6CNo ratings yet
- Review EQUILIBRIUM REVISIONDocument51 pagesReview EQUILIBRIUM REVISIONViper PotNo ratings yet
- Hill IRQDocument19 pagesHill IRQFrancisco Meneses FajardoNo ratings yet
- Chemical Kinetics 2Document39 pagesChemical Kinetics 2Md. Hasanur RahmanNo ratings yet
- Equilibrium Class XiDocument47 pagesEquilibrium Class XilololberuhlololNo ratings yet
- Chemical Reaction EqulibriumDocument9 pagesChemical Reaction EqulibriumRohan MehtaNo ratings yet
- LAS Week 2 GenChem2-Q2Document6 pagesLAS Week 2 GenChem2-Q2Drech LanadoNo ratings yet
- Ib Chem Topic 7 NotesDocument4 pagesIb Chem Topic 7 Notesseleem extraNo ratings yet
- Q4W2 616936307126976Document21 pagesQ4W2 616936307126976Jameel CailanNo ratings yet
- Handout No. 9 in General Chemistry 2: Co Qah + Melc LWDocument10 pagesHandout No. 9 in General Chemistry 2: Co Qah + Melc LWPortgas D. AceNo ratings yet
- Notes Equilibrium 1Document14 pagesNotes Equilibrium 1sanamaysha1No ratings yet
- Chemical Reaction EquilibriaDocument14 pagesChemical Reaction EquilibriaOmkar DolareNo ratings yet
- Chem 12 EquilibriumDocument31 pagesChem 12 EquilibriumryankyleacostaNo ratings yet
- Influence of EquilibriaDocument18 pagesInfluence of EquilibriaRejed VillanuevaNo ratings yet
- L1.Chemical Equilibrium. KUHES.2023Document84 pagesL1.Chemical Equilibrium. KUHES.2023DENACE JnrNo ratings yet
- CBSE Class 11 Chemistry-EquilibriumDocument71 pagesCBSE Class 11 Chemistry-EquilibriumkrkdjcjjddjNo ratings yet
- The Position of EquilibriumDocument2 pagesThe Position of Equilibriumusulasia777No ratings yet
- Chemical Engineering Process AnalysisDocument31 pagesChemical Engineering Process AnalysissepticmoneyNo ratings yet
- Topic 7Document9 pagesTopic 7Michelle EnkhsaikhanNo ratings yet
- Equilibrium NotesDocument3 pagesEquilibrium NotesSaumiaDevadasNo ratings yet
- Edexcel IAL Chemistry A-Level: Topic 13: Chemical EquilibriaDocument6 pagesEdexcel IAL Chemistry A-Level: Topic 13: Chemical EquilibriaMer CyNo ratings yet
- Fluid Mechanics Lab Report 1 AliDocument10 pagesFluid Mechanics Lab Report 1 AliAli ArshadNo ratings yet
- Astm D1238 23Document11 pagesAstm D1238 23agarciaNo ratings yet
- Leok 9Document2 pagesLeok 9Ali RumyNo ratings yet
- Experimental Study of Heat Transfer by Free Convection For Horizontal Cylinder of Composite Material Under The Effect of VibrationsDocument7 pagesExperimental Study of Heat Transfer by Free Convection For Horizontal Cylinder of Composite Material Under The Effect of VibrationsOfficial MusharafNo ratings yet
- Sizing Calculation - UPS System - SampleDocument1 pageSizing Calculation - UPS System - SampleDurgeshkumar RajputNo ratings yet
- Full Handbook Thermodynamics11005Document169 pagesFull Handbook Thermodynamics11005Max NighswanderNo ratings yet
- دورات LS linearDocument30 pagesدورات LS linearAli OmarNo ratings yet
- Cambridge IGCSE: Physics 0625/12Document16 pagesCambridge IGCSE: Physics 0625/12Muhammad Ejaz TariqNo ratings yet
- Name: - USF U-Number: - EGN3343: Thermodynamics I Midterm #1Document6 pagesName: - USF U-Number: - EGN3343: Thermodynamics I Midterm #1Bazal BhattiNo ratings yet
- Calculations Used in Analytical ChemistryDocument52 pagesCalculations Used in Analytical ChemistryNajibah A. CasimNo ratings yet
- OIML R 111-1 EspecificacionesDocument2 pagesOIML R 111-1 Especificacionessebastian perezNo ratings yet
- BS 2633-1987 PDFDocument58 pagesBS 2633-1987 PDFAndres Afanador MuñozNo ratings yet
- Lecture 14Document23 pagesLecture 14Ch Muhammad YousufNo ratings yet
- NMDCAT Books Set (PMC-2021)Document412 pagesNMDCAT Books Set (PMC-2021)PM-II-Alpha1-001 Fatima-tuz-Zahra100% (1)
- SAMPLE Examination in ELEMENTARY STATDocument2 pagesSAMPLE Examination in ELEMENTARY STATWindel Q. BojosNo ratings yet
- Special Lab Report Simple Pendulum Length Variation FinalDocument9 pagesSpecial Lab Report Simple Pendulum Length Variation Finalapi-541796478No ratings yet
- Numerical ApertureDocument8 pagesNumerical ApertureAryabrat MahapatraNo ratings yet
- Mock Ioqp Test 1 SolDocument20 pagesMock Ioqp Test 1 SolSantosh SinghNo ratings yet
- Sec 3 Chapter 5Document22 pagesSec 3 Chapter 5Edu 4 UNo ratings yet
- D445-21e1-VISCO ASTMDocument17 pagesD445-21e1-VISCO ASTMyulfanNo ratings yet
- (B.A.12) Rekayasa, 2021 14 (1) - Evaluasi Desain Bejana Bertekanan....Document7 pages(B.A.12) Rekayasa, 2021 14 (1) - Evaluasi Desain Bejana Bertekanan....M. SukarmanNo ratings yet
- LE-week-2 and 3 Lesson 2 Eim Quarter 3Document6 pagesLE-week-2 and 3 Lesson 2 Eim Quarter 3Aurelio LagunaNo ratings yet
- Problem Sets For Solutions AnalysisDocument2 pagesProblem Sets For Solutions AnalysisKamil Guillergan100% (1)
- HT Combitest 2019 SpecificationDocument3 pagesHT Combitest 2019 SpecificationAlbertofullHdNo ratings yet
- GENERAL PHYSICS 2 - Q3 - Week 4Document27 pagesGENERAL PHYSICS 2 - Q3 - Week 4ariinnggg onichaNo ratings yet
- SWRAUTOTESTERDocument14 pagesSWRAUTOTESTERslawekNo ratings yet
- Lab Activity 1: Verification of Ohm'S Law, KCL and KVL: ObjectivesDocument5 pagesLab Activity 1: Verification of Ohm'S Law, KCL and KVL: ObjectivesNeszel Jean Japus SaplotNo ratings yet
- AP Chemistry: Free-Response QuestionsDocument21 pagesAP Chemistry: Free-Response Questions소피아No ratings yet
- Rotation Solution: EXAMPLE 14: A 1200 LB Sled Is Pulled Along ADocument4 pagesRotation Solution: EXAMPLE 14: A 1200 LB Sled Is Pulled Along AJamael AbulaisNo ratings yet
- Physics MSCQDocument15 pagesPhysics MSCQkeshav GopaulNo ratings yet