Professional Documents
Culture Documents
Thermodynamics by Akansha Karnwal - Watermark
Thermodynamics by Akansha Karnwal - Watermark
Uploaded by
technicalfacts31Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Thermodynamics by Akansha Karnwal - Watermark
Thermodynamics by Akansha Karnwal - Watermark
Uploaded by
technicalfacts31Copyright:
Available Formats
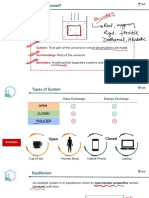
THERMODYNAMICS
THERMODYNAMICS PROPERTIES
PROCESSES
Isothermal Process INTENSIVE PROPERTIES EXTENSIVE PROPERTIES THERMODYNAMICS AKANSHA KARNWAL
ΔT = 0 P, T, N V, U, H
ZEROTH LAW OF THERMODYNAMICS
Isochoric Process Properties of the system Properties of the system GIBB'S ENERGY CHANGE
which are indepentent of which depend on the amount GIBB'S FREE ENERGY & EQUILIBRIUM
ΔV = 0 If two bodies are in
amount of system. of the system. A
Adiabatic Process STATE FUNCTION PATH FUNCTION
thermal equilibrium with The net energy available to do ΔG° = −2.303RT log K
q=0 Values of such functions Values of such functions a third one, then they useful work and it is a measure ΔG° = 0 log K = 0 Equilibrium reached.
do not depend on the path depend on the path of the are in thermal of spontanity.
Cyclic Process ΔG° < 0 log K > 1 Mixture contains
of the system. system. A A equilibrium with each
ΔU cyclic = 0 ΔG°= ΣΔG°Products −ΣΔG°Reactants mostly products.
e.g. ΔU, ΔH, ΔS etc. e.g. W and q other.
ΔG° > 0 log K < 1 Mixture contains
ΔG° = ΔH° - TΔS° mostly reactants.
TYPES OF SYSTEM
ΔG < 0, Process is spontaneous ΔG > 0, Process is non-spontaneous
OPEN SYSTEM ENTHALPY ΔG = 0, Process is at equilibrium
CLOSED SYSTEM
Matter
Energy
ΔH = ΔU + Δ(PV) Sign of Sign of
ISOTHERMAL IRREVERSIBLE ΔG = ΔH − TΔs Spontanity
Energy ΔH = ΔU + Δng RT WORK ΔH ΔS
Wirr= -Pext ΔV Negative Positive Always Negative Spontaneous at all temp.
ISOLATED SYSTEM Enthalpy of reaction (ΔH) ISOTHERMAL REVERSIBLE Positive Negative Always Positive Non-spontaneous at all temperature.
Matter ΔH = ΣΔH f(Products) - ΣΔH f(Reactants W
rev ( )
= -2.303nRT log
V2
V1 +ve @ low temp. Non-spontaneous at all temperature.
Positive Positive
Energy ΔH = ΣB.E. Reactants - ΣΒ.Ε Products W = -2.303nRT log (
rev ) P1
P2
-ve @ low temp. Spontaneous at high temperature.
ADIABATIC REVERSIBLE -ve @ low temp. Non-spontaneous at all temperature.
Enthalpy of fusion (ΔH°) Enthalpy change when 1 mole of compound undergoes melting at nR (T2-T1) Negative Negative
H O(s)→H O(l)
f Wre = +ve @ low temp. Spontaneous at high temperature.
2 2 constant temperature and atmospheric pressure. γR
Enthalpy of vapourization (ΔH° ) Enthalpy change when 1 mole of compound undergoes boiling at ENTROPY
vap
H O(l)→H O(g) constant temperature and atmospheric pressure. INTERNAL
2 2
HEAT (q) WORK (W) This is the measure of the degree of
Enthalpy of Sublimation (ΔH°sub ) Enthalpy change when 1 mole of solid substance is directly ENERGY (U) SPONTANEITY randomness or disorder of the system.
converted into gaseous state at a constant temperature and Work is a mode of energy
CO2 (s)→CO2 (g) Exchange of energy
standard pressure. Total energy within transfer when It is natural direction of a process. Δs =
q rev
due to temperature T
Enthalpy of Solution (ΔH°sol ) the substance temperature difference
Enthalpy change when 1 mole of substance is dissolved in fixed differnece.
KCl + aq→KCl (aq) quantity of solvent.
is not involved. ΔS =ΔS + ΔS
W = -PextΔV Total system surroundings
Bond dissociation enthalpy (Β.Ε.) It is the enthalpy change to break 1 mole bonds of a particular kind. FIRST LAW OF THERMODYNAMICS Entropy changes during phase transformation
Cl2 (g)→ 2Cl(g)
Law of conservation of energy total energy of an isolated
FREE EXPANSION ΔS fusion = ΔHfusion
, ΔS = ΔH vap
T vap T
HESS' LAW CONSTANT HEAT system is constant. When an ideal gas expands in vacuum then, pext= 0.
EORN HABER CYCLE
Molar HEAT CAPACITY Na+ (g) + Cl (g) Δ vap H
SUMMATION
Mathematically, Δu= q + w
∴ W=0 Δ
vap
S =
T
Amount of heat required to raise the
ΔH1 Na+(g) + 1 Cl(g) THIRD LAW OF Entropy change of a reaction.
temperature of a 1 mole substance.
A B SIGN CONVENTION
Molar heat capacity ⇒ qv = NvRT
2 -
Na+ (g) + Cl (g) SECOND LAW OF THERMODYNAMICS THERMODYNAMICS Δr S° = Σs°products − Σs°reactants
Specific heat capacity⇒ qp = nCpRT
+
Na (g) + 1 Cl(g) Heat absorbed by the Work done by the The Total entropy of the universe is always
akanshakarnwal_
ΔH ΔH = ΔH + ΔH + ΔH
1 2 3 ΔH2 2 system = -ve The Entropy of a perfectly crystalline
POISSON'S system = +ve increasing in the course of every spontaneous
MEYERS'S
RATIO Na+(s) + 1 Cl(g) substance at 0 K or absolute zero
FORMULA or natural change.
Cp - Cv = R
C p =γ D C 2 Heat evolved by the Work done on the
Temperature to be zero.
Cv ΔH3 NaCl(s) system = -ve system = +ve ΔS Total> 0
You might also like
- Solutions: Solutions Manual For Introduction To The Thermodynamics of Materials 6Th Edition GaskellDocument228 pagesSolutions: Solutions Manual For Introduction To The Thermodynamics of Materials 6Th Edition Gaskellhamed pirboneh75% (4)
- Air-Conditioning ExperimentDocument12 pagesAir-Conditioning ExperimentSerhat Güven0% (1)
- Chap 27 No 1Document2 pagesChap 27 No 1api-2638309040% (4)
- BS en 12390-14-2018 - (2020-11-10 - 07-43-02 PM) PDFDocument22 pagesBS en 12390-14-2018 - (2020-11-10 - 07-43-02 PM) PDFYuvaraj Dhandapani100% (1)
- Information/Data Required For Wax Modelling: A) B) C) D) E)Document6 pagesInformation/Data Required For Wax Modelling: A) B) C) D) E)AYAUWU LOVEDAY100% (1)
- Thermodynamics-1 MindmapDocument1 pageThermodynamics-1 Mindmapsarthakyedlawar04No ratings yet
- C6 ThermodynamicsDocument1 pageC6 ThermodynamicsPARAMBATH ANUP KUMARNo ratings yet
- Lecture 06 Biophysics Free EnergyDocument12 pagesLecture 06 Biophysics Free EnergyBelaliaNo ratings yet
- UntitledDocument36 pagesUntitledLmao DedNo ratings yet
- 6 - Gibbs Free Energy PDFDocument12 pages6 - Gibbs Free Energy PDFJey BlaQNo ratings yet
- Thermodynamics FormulasDocument1 pageThermodynamics FormulasShivam PathakNo ratings yet
- Module 5Document37 pagesModule 5Marklynnard BautistaNo ratings yet
- ThermodynamicsDocument34 pagesThermodynamicsAssdfNo ratings yet
- 40 Minutes ThermodynamicsDocument20 pages40 Minutes ThermodynamicsDheeraj dixitNo ratings yet
- Gibbs Free Energy Gibbs Free Energy: DG DH - TdsDocument8 pagesGibbs Free Energy Gibbs Free Energy: DG DH - TdsEmmanuel HoangNo ratings yet
- ThermodynamicsDocument33 pagesThermodynamicstoeshipahadiyaNo ratings yet
- Cet-Iv - MCQDocument6 pagesCet-Iv - MCQRohit Ramesh KaleNo ratings yet
- Gibbs Free Energy Calculation & Thermodynamic FeasibilityDocument20 pagesGibbs Free Energy Calculation & Thermodynamic Feasibilityyash bhutadaNo ratings yet
- ThermodynamicsDocument9 pagesThermodynamicsJohn Bernard VillarosaNo ratings yet
- Chemical Thermodynamics - Wk7Document25 pagesChemical Thermodynamics - Wk7Hadassah ImbansNo ratings yet
- Thermo FundamentalsDocument75 pagesThermo FundamentalsJunaid Bin KhalidNo ratings yet
- The Third Law of Thermodynamics2Document30 pagesThe Third Law of Thermodynamics2Ivvani Aulia PutriNo ratings yet
- ThermodynamicsDocument8 pagesThermodynamicsharshillbhartiNo ratings yet
- 2nd Law of Thermodynamics Group 1 NebresDocument35 pages2nd Law of Thermodynamics Group 1 NebresrbxwmnNo ratings yet
- First Law Analysis of Non-Flow ProcessesDocument22 pagesFirst Law Analysis of Non-Flow ProcessesDeepak sainiNo ratings yet
- Lecture 4 March 2023Document21 pagesLecture 4 March 2023Gabriel Burgos KimNo ratings yet
- Revision Notes On Chemical ThermodynamicsDocument6 pagesRevision Notes On Chemical ThermodynamicsManish SainiNo ratings yet
- Peter Atkins Julio de Paula Ron Friedman Physical Chemistry Quanta (0664-0714)Document51 pagesPeter Atkins Julio de Paula Ron Friedman Physical Chemistry Quanta (0664-0714)Administracion OTIC IVICNo ratings yet
- Free EnergyDocument24 pagesFree EnergyVivek PattanashettiNo ratings yet
- RC DemoDocument16 pagesRC DemoAniket SharmaNo ratings yet
- Chapter 20 (Entropy & Free Energy)Document7 pagesChapter 20 (Entropy & Free Energy)Richard KimNo ratings yet
- Lecture 1introduction - Revision 18-8-2020Document41 pagesLecture 1introduction - Revision 18-8-2020Hamza AliNo ratings yet
- Thermodynamics 2 PDFDocument31 pagesThermodynamics 2 PDFAhmed Gahlan AlmaqzengyNo ratings yet
- Activity 2Document10 pagesActivity 2shukrani kasaseNo ratings yet
- asset-v1-DelftX+TP102x+3T2016+type@asset+block@Formula Sheet ATPDocument12 pagesasset-v1-DelftX+TP102x+3T2016+type@asset+block@Formula Sheet ATPkennethmsorianoNo ratings yet
- CHM432 Fundamental Physical Chemistry: ThermodynamicsDocument97 pagesCHM432 Fundamental Physical Chemistry: ThermodynamicsPriscyyNo ratings yet
- L03-Thermo - and - KineticsDocument15 pagesL03-Thermo - and - KineticsNgọc DươngNo ratings yet
- ThermodynamicsDocument9 pagesThermodynamicslopomat667No ratings yet
- ThermodynamicsDocument31 pagesThermodynamicsDebayanbasu.juNo ratings yet
- 28 - 7-PDF - Thermodynamics (Red Book) 2Document1 page28 - 7-PDF - Thermodynamics (Red Book) 2PranayNo ratings yet
- Appendix A: A1 Automatic Temperature CompensationDocument2 pagesAppendix A: A1 Automatic Temperature CompensationSur VaniNo ratings yet
- CH2105 - ThermodynamicsDocument12 pagesCH2105 - ThermodynamicsJohnNo ratings yet
- Ilovepdf Merged RemovedDocument144 pagesIlovepdf Merged RemovedRajeshNo ratings yet
- The Basis of Chemical Thermodynamics: Cai Zheng School of PharmacyDocument38 pagesThe Basis of Chemical Thermodynamics: Cai Zheng School of PharmacyBridget Hope LiuNo ratings yet
- Electrochemical Thermodynamics and Potentials LiSA 101 BoettcherDocument53 pagesElectrochemical Thermodynamics and Potentials LiSA 101 BoettcherBehruz ArghavaniNo ratings yet
- Thermodynamics I 2Document32 pagesThermodynamics I 2SamerNo ratings yet
- Thermodynamics WorksheetDocument13 pagesThermodynamics WorksheetHudsun HornetNo ratings yet
- Product RD Session 16 - Phase Eqm Part 3Document38 pagesProduct RD Session 16 - Phase Eqm Part 3Rishabh JainNo ratings yet
- Lecture 4 - Free EnergyDocument16 pagesLecture 4 - Free EnergyyudhiprasetyoNo ratings yet
- 65673556ebbd5a00188e56ad - ## - Thermodynamics Short N - 231217 - 235549Document3 pages65673556ebbd5a00188e56ad - ## - Thermodynamics Short N - 231217 - 235549shivrajcma007No ratings yet
- Thermodynamics Btech NEWDocument30 pagesThermodynamics Btech NEWAmol BharadwajNo ratings yet
- CH1201-TD-1st LawDocument20 pagesCH1201-TD-1st LawAbhroNo ratings yet
- 64b82d9803e1980018a78d67 - ## - 06 - Thermodynamics Short NotesDocument3 pages64b82d9803e1980018a78d67 - ## - 06 - Thermodynamics Short Noteskanishkagupta350No ratings yet
- Chapter 2: Some Concepts and Definitions: ThermodynamicsDocument66 pagesChapter 2: Some Concepts and Definitions: ThermodynamicsTanoosh KancharlaNo ratings yet
- MTA1-Sistemas, Propiedades, EnergíaDocument32 pagesMTA1-Sistemas, Propiedades, EnergíaJavier Noriega BarrientosNo ratings yet
- EPS ThermodynamicsDocument6 pagesEPS ThermodynamicsEmreNo ratings yet
- Physical Cheeeemistry 1Document49 pagesPhysical Cheeeemistry 1Parth SonawaneNo ratings yet
- Thermodynamic Part 1Document24 pagesThermodynamic Part 1Ad Man GeTigNo ratings yet
- 1st Law: Conservation of EnergyDocument85 pages1st Law: Conservation of EnergyAyu MilineaNo ratings yet
- Thermodynamics FormulasDocument3 pagesThermodynamics FormulasZack D. SnutssNo ratings yet
- Thermodynamics S8 211Document238 pagesThermodynamics S8 211Avijeet NaiyaNo ratings yet
- Chapter 4 - 32044472 - 2024 - 03 - 26 - 17 - 48Document7 pagesChapter 4 - 32044472 - 2024 - 03 - 26 - 17 - 48samyakpande1008No ratings yet
- TD 2 EnthuseDocument43 pagesTD 2 Enthuseipsita lahiriNo ratings yet
- Biological Classification MoneraDocument21 pagesBiological Classification Moneratechnicalfacts31No ratings yet
- Chemical BondingDocument12 pagesChemical Bondingtechnicalfacts31No ratings yet
- SRG SPS-06 17 Nov SoDocument3 pagesSRG SPS-06 17 Nov Sotechnicalfacts31No ratings yet
- SRG SPS-03 07 Nov SoDocument3 pagesSRG SPS-03 07 Nov Sotechnicalfacts31No ratings yet
- SRG SPS-08 22 Nov SoDocument4 pagesSRG SPS-08 22 Nov Sotechnicalfacts31No ratings yet
- SRG SPS-02 06 Nov SoDocument3 pagesSRG SPS-02 06 Nov Sotechnicalfacts31No ratings yet
- SRG SPS-04 08 Nov SoDocument3 pagesSRG SPS-04 08 Nov Sotechnicalfacts31No ratings yet
- SRG SPS-01 04 Nov SoDocument3 pagesSRG SPS-01 04 Nov Sotechnicalfacts31No ratings yet
- SRG Minor-17 (C) 09 Aug QPDocument20 pagesSRG Minor-17 (C) 09 Aug QPtechnicalfacts31No ratings yet
- YCT Moving Charges & Magnetism NEET JEE Practice QuestionsDocument189 pagesYCT Moving Charges & Magnetism NEET JEE Practice Questionstechnicalfacts31100% (1)
- YCT Wave Optics NEET JEE Practice QuestionsDocument117 pagesYCT Wave Optics NEET JEE Practice Questionstechnicalfacts31100% (1)
- YCT Capacitance NEET JEE Questions PracticeDocument152 pagesYCT Capacitance NEET JEE Questions Practicetechnicalfacts31100% (1)
- Power Generation Using Multi Component Working FluidsDocument31 pagesPower Generation Using Multi Component Working FluidsaroontpeNo ratings yet
- Pipe Preboard 1Document10 pagesPipe Preboard 1Chyno Kang100% (1)
- KInetic Model of Matter WorksheetDocument7 pagesKInetic Model of Matter WorksheetNudrat50% (2)
- Variable Refrigerant Flow Systems VRF 11Document56 pagesVariable Refrigerant Flow Systems VRF 11Engineering WavesNo ratings yet
- Physics,: MechanicsDocument8 pagesPhysics,: Mechanicsdarwin delatorreNo ratings yet
- 4 8 - FprENDocument61 pages4 8 - FprENraduvlasaNo ratings yet
- Termodinamika Technique Jilid 1 Edition 4Document5 pagesTermodinamika Technique Jilid 1 Edition 4NholisbarberNo ratings yet
- Mix The Heat PDFDocument2 pagesMix The Heat PDFLaine AcainNo ratings yet
- 16 Heats of Formation - SDocument5 pages16 Heats of Formation - Sapi-313691183100% (1)
- Ipd-Module IiDocument10 pagesIpd-Module Iijohn babe jeszareth CapiliNo ratings yet
- Problem Set 6 SolutionDocument4 pagesProblem Set 6 SolutionRod De GuzmanNo ratings yet
- General Ashg07kmtaDocument35 pagesGeneral Ashg07kmtaFrancisco Martin BurgosNo ratings yet
- Chemical Thermodynamics Y: David A. KatzDocument44 pagesChemical Thermodynamics Y: David A. Katztheodore_estradaNo ratings yet
- Column of Industrial Solvent Recovery Distillation ColumnDocument7 pagesColumn of Industrial Solvent Recovery Distillation Columndhavalesh1No ratings yet
- Manual SalinasDocument90 pagesManual SalinasRenan AlvesNo ratings yet
- Heat Exchangers and It'S Classification, Temperature Disruibution in Heat ExangersDocument15 pagesHeat Exchangers and It'S Classification, Temperature Disruibution in Heat Exangersdhyan shahNo ratings yet
- Oct 2010 ASHRAE PICV PresentationDocument45 pagesOct 2010 ASHRAE PICV PresentationJaved BhattiNo ratings yet
- Latent Heat of Vaporization Via Hysys Flash Calculations - LinkedInDocument5 pagesLatent Heat of Vaporization Via Hysys Flash Calculations - LinkedIngopinath87No ratings yet
- Manual Chiller 10 14Document61 pagesManual Chiller 10 14Daanii OsoRiio100% (1)
- Calorimetry ProblemsDocument2 pagesCalorimetry ProblemsSid Damien TanNo ratings yet
- Heavy-Duty Heating, Ventilation, and Air Conditioning SystemsDocument57 pagesHeavy-Duty Heating, Ventilation, and Air Conditioning SystemsNezar Amin0% (1)
- Problem Set 9Document4 pagesProblem Set 9Ykhay ElfanteNo ratings yet
- 13 Radiative Heat Transfer PDFDocument11 pages13 Radiative Heat Transfer PDFpremanth reddyNo ratings yet
- APH Seal LeakagesDocument17 pagesAPH Seal LeakagesBhargav ChaudhariNo ratings yet
- Hydronics Application ManualDocument56 pagesHydronics Application ManualGuy Blouin100% (1)