Professional Documents
Culture Documents
C6 Thermodynamics
C6 Thermodynamics
Uploaded by
PARAMBATH ANUP KUMAROriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
C6 Thermodynamics
C6 Thermodynamics
Uploaded by
PARAMBATH ANUP KUMARCopyright:
Available Formats
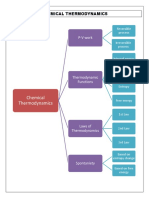
THERMODYNAMICS
PROCESSES
THERMODYNAMICS PROPERTIES THERMODYNAMICS GIBB'S FREE ENERGY
The net energy available to do useful
GIBB'S ENERGY CHANGE
& EQUILIBRIUM
• Isothermal Process work and it is a measure of ∆G° = −2.303RT log K
∆T = 0
INTENSIVE PROPERTIES EXTENSIVE PROPERTIES spontanity. ∆G° = 0 log K = 0 Equilibrium reached.
P, T, N
ZEROTH LAW OF THERMODYNAMICS Standard free energy of a reaction:
• Isochoric Process V, U, H
∆G°= Σ∆G°Products −Σ∆G°Reactants ∆G° < 0 log K > 1 Mixture contains
∆V = 0 Properties of the system Properties of the system
which are indepentent of If two bodies are in mostly products.
which depend on the amount
amount of system. A thermal equilibrium with ∆G° = ∆H° - T∆S° ∆G° > 0 log K < 1 Mixture contains
• Adiabatic Process of the system.
a third one, then they
mostly reactants.
q=0 STATE FUNCTION PATH FUNCTION
Values of such functions Values of such functions are in thermal
• Cyclic Process do not depend on the path
of the system.
depend on the path of the
system.
equilibrium with each ∆G < 0, Process is spontaneous ∆G > 0, Process is non-spontaneous
A A
∆Ucyclic = 0 other.
e.g. ∆U, ∆H, ∆S etc. e.g. W and q .
∆G = 0, Process is at equilibrium
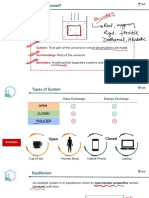
TYPES OF SYSTEM Sign of Sign of ∆ G = ∆ H − T∆ s Spontanity.
ISOTHERMAL IRREVERSIBLE ∆H ∆S
OPEN SYSTEM CLOSED SYSTEM WORK
Always
Negative Positive Spontaneous at all temp.
Wirr = -Pext ∆V Negative
Matter
ENTHALPY Positive Negative
Always Non-spontaneous at all
ISOTHERMAL REVERSIBLE Positive temperature.
Energy Energy Non-spontaneous at all
V2 +ve @ low temp.
Wrev = -2.303nRT log temperature.
∆H = ∆U + ∆(PV) V1 Postive Postive
spontaneous at high
-ve @ low temp.
∆H = ∆U + ∆ngRT P1 temperature.
ISOLATED SYSTEM Wrev = -2.303nRT log
P2 -ve @ low temp. spontaneous at low
Enthalpy of reaction (∆H) Negative Negative
temperature.
spontaneous at low
ADIABATIC REVERSIBLE +ve @ low temp.
Matter ∆H = Σ∆Hf(Products) - Σ∆Hf(Reactants) temperature.
nR (T -T )
Energy Wrev =
∆H = ΣB.E.Reactants - ΣΒ.ΕProducts γR 2 1
SPONTANEITY
Enthalpy of fusion (∆H°f) Enthalpy change when 1 mole of compound undergoes melting at WORK (W) It is natural direction of a process.
H 2 O(s) → H 2 O(l) constant temperature and atmospheric pressure.
HEAT (q) INTERNAL Work is a mode of energy
Enthalpy of vapourization (∆H°vap) Enthalpy change when 1 mole of compound undergoes boiling at ENERGY (U) transfer when
H 2 O() → H 2 O(g) constant temperature and atmospheric pressure. temperature difference
Exchange of energy
due to temperature Total energy within is not involved.
Enthalpy of Sublimation (∆H°sub) Enthalpy change when 1 mole of solid substance is directly converted differnece. the substance W = -Pext∆V
CO 2 (s) → CO 2 (g)
into gaseous state at a constant temperature and standard
pressure. ENTROPY
Enthalpy of Solution (∆H°sol) Enthalpy change when 1 mole of substance is dissolved in fixed FIRST LAW OF THERMODYNAMICS This is the measure of the degree of
KCl + aq → KCl (aq)
quantity of solvent. randomness or disorder of the system.
q rev
Law of conservation of energy total energy of an isolated ∆s =
T
Bond dissociation enthalpy (Β.Ε.) It is the enthalpy change to break 1 mole bonds of a particular kind. system is constant.
Cl2(g) 2Cl(g) Mathematically, ∆u= q + w ∆STotal = ∆S system
+ ∆S surroundings
Entropy changes during phase transformation
FREE EXPANSION ∆Hfusion ∆Hvap
SIGN ∆Sfusion= , ∆Svap=
Molar HEAT CAPACITY HESS' LAW CONSTANT HEAT Na+ (g) + Cl (g) T T
CONVENTION
EORN HABER CYCLE
When an ideal gas expands in vacuum ∆ H
SUMMATION then, pext = 0. ∆ sub S = subs
Amount of heat required to raise the 12∆bondH° T
Heat absorbed by the ∴ W=0
Na+(g) + 1 Cl(g) Entropy change of a reaction.
temperature of a 1 mole substance. ∆egH°
∆H1 system = +ve
A B 2
Molar heat capacity ⇒ qv = NvRT ∆ r S° = Σ s°products − Σ s°reactants
∆iH° Heat evolved by the
Specific heat capacity ⇒ qp = nCpRT 1
Na (g) + Cl(g)
+ Na+ (g) + Cl (g) -
system = -ve
SECOND LAW OF
∆H ∆H2 2 THERMODYNAMICS
MEYERS'S POISSON'S ∆subH° Work done by the THIRD LAW OF
RATIO ∆H3 system = -ve The Total entropy of the universe is always THERMODYNAMICS
FORMULA D C Na (s) + 1 Cl(g)
+ ∆LatticeH° increasing in the course of every s-
Cp 2
Cp - Cv = R =γ ∆fH°
Work done on the pontaneous or natural change. The Entropy of a perfectly crystalline
Cv ∆H = ∆H1 + ∆H2 + ∆H3
system = +ve
∆STotal > 0
substance at 0 K or absolute zero
Temperature to be zero.
NaCl(s)
anand_mani16 DR. Anand Mani https://www.anandmani.com/ https://discord.io/anandmani t.me/anandmani001
You might also like
- Chapter 4 Diode CircuitsDocument140 pagesChapter 4 Diode Circuitsshubhankar palNo ratings yet
- MD1 Assignment 2 MT Period Clutches TongcoDocument3 pagesMD1 Assignment 2 MT Period Clutches TongcoLister NambatacNo ratings yet
- Advanced Engineering Thermodynamics: Thermodynamics and Fluid Mechanics SeriesFrom EverandAdvanced Engineering Thermodynamics: Thermodynamics and Fluid Mechanics SeriesRating: 4 out of 5 stars4/5 (9)
- Thermodynamics by Akansha Karnwal - WatermarkDocument1 pageThermodynamics by Akansha Karnwal - Watermarktechnicalfacts31No ratings yet
- Thermodynamics-1 MindmapDocument1 pageThermodynamics-1 Mindmapsarthakyedlawar04No ratings yet
- The Third Law of ThermodynamicsDocument8 pagesThe Third Law of ThermodynamicsJOHN ROLIE MAMELOCONo ratings yet
- First Law Analysis of Non-Flow ProcessesDocument22 pagesFirst Law Analysis of Non-Flow ProcessesDeepak sainiNo ratings yet
- Thermo FundamentalsDocument75 pagesThermo FundamentalsJunaid Bin KhalidNo ratings yet
- Modelling of Energy in Transit Using PythonDocument5 pagesModelling of Energy in Transit Using PythonInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- CH 15d-WDYEADocument53 pagesCH 15d-WDYEAktmbych2016No ratings yet
- Elements of Mechanics IntorDocument47 pagesElements of Mechanics IntorRenel AluciljaNo ratings yet
- C AIMS DTS@Chemical 20thermodynamic 20and 20enerjetics 20bDocument13 pagesC AIMS DTS@Chemical 20thermodynamic 20and 20enerjetics 20bAditya RamNo ratings yet
- MTA1-Sistemas, Propiedades, EnergíaDocument32 pagesMTA1-Sistemas, Propiedades, EnergíaJavier Noriega BarrientosNo ratings yet
- TD-6 - First Law-Open SystemDocument20 pagesTD-6 - First Law-Open SystemAhmed HassanNo ratings yet
- CH 15d-WDYEADocument55 pagesCH 15d-WDYEAValerie Duran-ArzagaNo ratings yet
- 06 ThermodynamicsDocument26 pages06 ThermodynamicsErfan Abbas zadeh BazziNo ratings yet
- 3.hafta 1Document16 pages3.hafta 1woboc79249No ratings yet
- Chapter - 01 - Basic ConceptsDocument34 pagesChapter - 01 - Basic ConceptsJeric MangubatNo ratings yet
- Electrochemical Thermodynamics and Potentials LiSA 101 BoettcherDocument53 pagesElectrochemical Thermodynamics and Potentials LiSA 101 BoettcherBehruz ArghavaniNo ratings yet
- Lecture 01 Fundamentals of Thermodynamics 1aDocument28 pagesLecture 01 Fundamentals of Thermodynamics 1aIsmael Torres-PizarroNo ratings yet
- CHM432 Fundamental Physical Chemistry: ThermodynamicsDocument97 pagesCHM432 Fundamental Physical Chemistry: ThermodynamicsPriscyyNo ratings yet
- Adiabatic and Isothermal ProcessesDocument2 pagesAdiabatic and Isothermal ProcessesphydotsiNo ratings yet
- Introduction & Basic Concepts of Thermodynamics: Reading ProblemsDocument6 pagesIntroduction & Basic Concepts of Thermodynamics: Reading Problemstpjoshi1No ratings yet
- Ilovepdf Merged RemovedDocument144 pagesIlovepdf Merged RemovedRajeshNo ratings yet
- EXP 1 - Physics Finals PDFDocument10 pagesEXP 1 - Physics Finals PDFPearl ArcamoNo ratings yet
- Chemical Engineering Thermodynamics: Unit 1 Fundamental Concepts and The First Law of ThermodynamicsDocument42 pagesChemical Engineering Thermodynamics: Unit 1 Fundamental Concepts and The First Law of ThermodynamicsdonigNo ratings yet
- Introduction and Basic Concepts Study Guide in Powerpoint: To AccompanyDocument34 pagesIntroduction and Basic Concepts Study Guide in Powerpoint: To AccompanyDuy NguyenNo ratings yet
- Thermodynamics 2 (TRDMIA2)Document24 pagesThermodynamics 2 (TRDMIA2)Njabulo MdlaloseNo ratings yet
- Chapter 2: Some Concepts and Definitions: ThermodynamicsDocument66 pagesChapter 2: Some Concepts and Definitions: ThermodynamicsTanoosh KancharlaNo ratings yet
- An Autonomous InstituteDocument80 pagesAn Autonomous InstituteayeshaNo ratings yet
- ThermodynamicsDocument67 pagesThermodynamicsHimanshu RaiNo ratings yet
- Chapter 1Document34 pagesChapter 1parsa.shakeri.bavilNo ratings yet
- Thermodynamics S8 211Document238 pagesThermodynamics S8 211Avijeet NaiyaNo ratings yet
- UntitledDocument36 pagesUntitledLmao DedNo ratings yet
- Termodinamika Teknik Kimia 2 - Minggu 1Document29 pagesTermodinamika Teknik Kimia 2 - Minggu 1SandyPratamaAdhiNo ratings yet
- 3 Thermodynamics TDEEDocument129 pages3 Thermodynamics TDEENikhilNo ratings yet
- Thermodynamics Module 1Document12 pagesThermodynamics Module 1Kirtan KumarNo ratings yet
- Thermodynamic GlossaryDocument3 pagesThermodynamic GlossaryAdrian ArroyoNo ratings yet
- Ch1 ThermochemistryDocument9 pagesCh1 ThermochemistryRonnel ClarinNo ratings yet
- ThermodynamicsDocument34 pagesThermodynamicsAssdfNo ratings yet
- Exergy: TermodynamicsDocument13 pagesExergy: TermodynamicsMokhammad Fahmi IzdiharrudinNo ratings yet
- Thermodynamics Btech NEWDocument30 pagesThermodynamics Btech NEWAmol BharadwajNo ratings yet
- Reu2015 Winston ChuDocument1 pageReu2015 Winston ChuaptridioNo ratings yet
- Chapter 7 - EntropyDocument50 pagesChapter 7 - EntropyKrishh GuptaNo ratings yet
- Energy Work Change: Definitions CapacityDocument12 pagesEnergy Work Change: Definitions CapacityPei WuNo ratings yet
- Topic: Fundamentals of Thermodynamics Subject: ThermodynamicsDocument24 pagesTopic: Fundamentals of Thermodynamics Subject: Thermodynamicsbharathkumar0310No ratings yet
- ThermodynamicsDocument86 pagesThermodynamicsmithunkrish2222No ratings yet
- Thermodynamics V Jee 2024Document163 pagesThermodynamics V Jee 2024visheshbindal2007No ratings yet
- Chemical Thermodynamics - Wk7Document25 pagesChemical Thermodynamics - Wk7Hadassah ImbansNo ratings yet
- 03 Chapter 04 - Part 1 - 05Document41 pages03 Chapter 04 - Part 1 - 05MmmmohNo ratings yet
- Chapter 15 - WavesDocument10 pagesChapter 15 - WavesgnkstarNo ratings yet
- MTG Fluidized Bed Reactor-Regenerator Unit With Catalyst CirculationDocument13 pagesMTG Fluidized Bed Reactor-Regenerator Unit With Catalyst CirculationLuana ArêbaNo ratings yet
- CH02 Thermodynamics For BMEngDocument21 pagesCH02 Thermodynamics For BMEngEyob FikiraddisNo ratings yet
- Activity 2Document10 pagesActivity 2shukrani kasaseNo ratings yet
- ME 306 Part 4 Compressible FlowDocument15 pagesME 306 Part 4 Compressible FlowBeke derejeNo ratings yet
- Chapter 2: Some Concepts and Definitions: ThermodynamicsDocument53 pagesChapter 2: Some Concepts and Definitions: ThermodynamicsNanda KumarNo ratings yet
- Steam EngineDocument48 pagesSteam EngineReeyan Dave DagandanNo ratings yet
- Thermo - PHD Week 1Document27 pagesThermo - PHD Week 1HardiNo ratings yet
- Thermo SummaryDocument42 pagesThermo SummaryManoranjan Kumar SinghNo ratings yet
- Proposal Fung Fabio - 00Document14 pagesProposal Fung Fabio - 00Daryll ShawnNo ratings yet
- C4 Chemical KineticsDocument1 pageC4 Chemical KineticsPARAMBATH ANUP KUMARNo ratings yet
- 7 EquilibriumDocument1 page7 EquilibriumPARAMBATH ANUP KUMARNo ratings yet
- A13 Hydrocarbons NewDocument1 pageA13 Hydrocarbons NewPARAMBATH ANUP KUMARNo ratings yet
- C9 Coordination CompoundsDocument1 pageC9 Coordination CompoundsPARAMBATH ANUP KUMARNo ratings yet
- C13 AminesDocument1 pageC13 AminesPARAMBATH ANUP KUMARNo ratings yet
- Aldehyde Ketones and Carboxylic AcidDocument1 pageAldehyde Ketones and Carboxylic AcidPARAMBATH ANUP KUMARNo ratings yet
- Organic Chemistry Basics Some PrincipleDocument1 pageOrganic Chemistry Basics Some PrinciplePARAMBATH ANUP KUMARNo ratings yet
- Surface ChemistryDocument1 pageSurface ChemistryPARAMBATH ANUP KUMARNo ratings yet
- Redox ReactionDocument1 pageRedox ReactionPARAMBATH ANUP KUMARNo ratings yet
- AIITS 2123 OT JEEA Paper 2Document13 pagesAIITS 2123 OT JEEA Paper 2Beastly BeatleNo ratings yet
- CapacitorsDocument12 pagesCapacitorsAndrei NicolaeNo ratings yet
- Design and Development of An External Fast Neutron BeamDocument125 pagesDesign and Development of An External Fast Neutron BeamFadhel HmoodNo ratings yet
- Set Soalan PT3Document35 pagesSet Soalan PT3Anthony Hunt100% (1)
- Lect 6 P Channel JFETDocument9 pagesLect 6 P Channel JFETHari Mohan RaiNo ratings yet
- Date of 1 Ed: 21.02.2013 Name: TD-SEH / D. Gautschi Ed. No. 5, 31.07.2015 © ALSTOM Grid AG, OberentfeldenDocument2 pagesDate of 1 Ed: 21.02.2013 Name: TD-SEH / D. Gautschi Ed. No. 5, 31.07.2015 © ALSTOM Grid AG, OberentfeldenRinda_RaynaNo ratings yet
- Prasanna Sajee D & F Block Elements: Class 12 - ChemistryDocument3 pagesPrasanna Sajee D & F Block Elements: Class 12 - ChemistryFaiz SyedNo ratings yet
- Pulsation of Flow and Pressure in Piping of Reciprocating CompressorsDocument26 pagesPulsation of Flow and Pressure in Piping of Reciprocating CompressorsSuhas JoshiNo ratings yet
- Suspended CeilingsDocument10 pagesSuspended CeilingsMaría Fernanda MataNo ratings yet
- Travel Transmission - Revised (Compatibility Mode)Document4 pagesTravel Transmission - Revised (Compatibility Mode)Wahab AbdulNo ratings yet
- AlkeneDocument2 pagesAlkeneGapor examNo ratings yet
- Application of System of Linear Equations 2018Document42 pagesApplication of System of Linear Equations 2018VuKhang NguyenNo ratings yet
- Ernest Rutherford's Two Major Contributions - 211007 - 130717Document8 pagesErnest Rutherford's Two Major Contributions - 211007 - 130717Hisagi ShuheiNo ratings yet
- GFRG Fiil Frsil: R (FFD Qitfe TRC Ener (Er (QfiDocument2 pagesGFRG Fiil Frsil: R (FFD Qitfe TRC Ener (Er (QfisrihariNo ratings yet
- TN-F701-12 - Evaluation of Screw StrengthDocument4 pagesTN-F701-12 - Evaluation of Screw StrengthDavid ArndtNo ratings yet
- Presentation Title Mundra UMPP: Presentation Subtitle CGPL, Tata Power Company LimitedDocument34 pagesPresentation Title Mundra UMPP: Presentation Subtitle CGPL, Tata Power Company LimitedJay JobanputraNo ratings yet
- FRQ Modern PhysicsDocument22 pagesFRQ Modern PhysicsIvycrazeNo ratings yet
- TND-AA PerformanceDocument1 pageTND-AA Performancehector tosarNo ratings yet
- Hladnjaca DefrostingDocument6 pagesHladnjaca DefrostingDzana KadricNo ratings yet
- APsystems Datasheet YC600 2019 ENDocument2 pagesAPsystems Datasheet YC600 2019 ENMarcelo SilvaNo ratings yet
- LINES AND ANGLES Class 9 CBSEDocument8 pagesLINES AND ANGLES Class 9 CBSEriya rajputNo ratings yet
- Lecture - 8 Notes Structure of AtomDocument2 pagesLecture - 8 Notes Structure of Atomrahul tudNo ratings yet
- Green Light HPS Laser Operator ManualDocument81 pagesGreen Light HPS Laser Operator ManualPhillip V Mitchell0% (1)
- Manual 875 KF Gas AnalyzerDocument49 pagesManual 875 KF Gas AnalyzerJack TranNo ratings yet
- Xerox WorkCentre 5016 5020 Service ManualDocument402 pagesXerox WorkCentre 5016 5020 Service ManualKlema Hanis83% (6)
- Daikin Summary - 18SEER 18K&24KDocument15 pagesDaikin Summary - 18SEER 18K&24KBernie AguilarNo ratings yet
- Dagon UniversityDocument6 pagesDagon UniversityMoe TheintNo ratings yet
- 2021 Sarawak - SMK Physics K1 - K2 JawapanDocument8 pages2021 Sarawak - SMK Physics K1 - K2 JawapanJasrul JamalNo ratings yet