0% found this document useful (0 votes)
4K views12 pagesQuantum Numbers
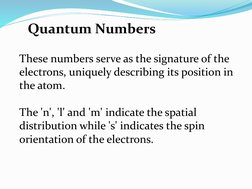
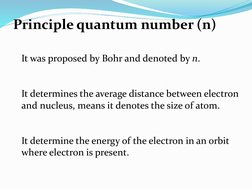
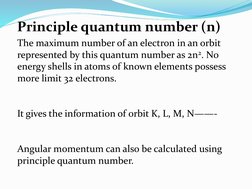
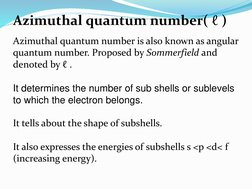
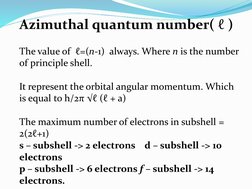
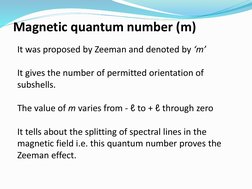
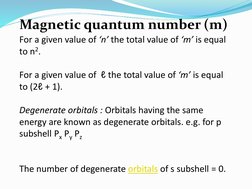
The quantum numbers n, l, m, and s uniquely define an electron's position in an atom. The principal quantum number n determines the electron's energy level or shell. The azimuthal quantum number l indicates the subshell, related to its shape. The magnetic quantum number m gives the subshell's orientation. The spin quantum number s represents the electron's intrinsic spin as either +1/2 or -1/2. Together these quantum numbers provide a signature for each atomic orbital.
Uploaded by
Soub kuopCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PPTX, PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
4K views12 pagesQuantum Numbers
The quantum numbers n, l, m, and s uniquely define an electron's position in an atom. The principal quantum number n determines the electron's energy level or shell. The azimuthal quantum number l indicates the subshell, related to its shape. The magnetic quantum number m gives the subshell's orientation. The spin quantum number s represents the electron's intrinsic spin as either +1/2 or -1/2. Together these quantum numbers provide a signature for each atomic orbital.
Uploaded by
Soub kuopCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PPTX, PDF, TXT or read online on Scribd
- Quantum Numbers
- Principle Quantum Number (n)
- Azimuthal Quantum Number (ℓ)
- Magnetic Quantum Number (m)
- Spin Quantum Numbers (s)