Professional Documents
Culture Documents
ANTACIDS
Uploaded by
shaitabligan0 ratings0% found this document useful (0 votes)
9 views7 pagesCopyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
9 views7 pagesANTACIDS
Uploaded by
shaitabliganCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 7
ANTACIDS
• Antacids are weak bases that are used to NEUTRALIZE
excess stomach acid
• Most antacids are weak inorganic bases
• Antacids DO NOT prevent
the over-production of acid
• Antacids DO neutralize the
• cid once it’s in the stomach
MECHANISM OF ACTION
• There is a certain level of acid in your stomach. A
system called the pH (potential of hydrogen) system
measures this level on a scale from 0-14.
• The normal acid level in the stomach is about 2 or 3
on this scale. A pH of 7 is neutral, below 7 is acid and
above 7 is alkaline.
• When there is excess acid in your stomach, the pH
level has probably dropped below the normal level of
2 or 3, and the job of the antacid, which is a base (the
opposite of an acid), is to neutralize some of the
excess acid.
Promote the gastric mucosal defense mechanisms:
Secretions of:
• Mucus: protective barrier against HCl.
• Bicarbonate: Helps buffer acidic properties of HCl
• Prostaglandin: Prevent activation of pump inhibitors
Common examples include
Calcium carbonate (CaCO3) Sodium bicarbonate (NaHCO3)
constipation, gas (flatulence) Nausea, bloating, or gas
Aluminum hydroxide (Al(OH)3) Magnesium hydroxide (Mg(OH)2)
MgO and Mg(OH)2 (Milk of Magnesia)
severe stomach pain or constipation diarrhea
Combined Antacids
Special Considerations
People with heart failure may have sodium restrictions to help decrease
fluid buildup. However, antacids often contain a lot of sodium.
People with kidney failure may develop a buildup of aluminum after
using antacids. This can lead to aluminum toxicity. People with kidney
failure also tend to have problems with electrolyte balance. All
antacids contain electrolytes, which could make electrolyte balance
problems worse.
You might also like
- Trends Affecting NSG PracticeDocument22 pagesTrends Affecting NSG PracticeshaitabliganNo ratings yet
- Antiemetic DrugsDocument21 pagesAntiemetic DrugsshaitabliganNo ratings yet
- Anti-Spasmodic DrugsDocument8 pagesAnti-Spasmodic DrugsshaitabliganNo ratings yet
- Proclamation No. 499Document21 pagesProclamation No. 499shaitabliganNo ratings yet
- Anti DiarrhealDocument5 pagesAnti DiarrhealshaitabliganNo ratings yet
- Cephalosphorins 3rd Gen 4rt GenDocument24 pagesCephalosphorins 3rd Gen 4rt GenshaitabliganNo ratings yet
- PenicillinDocument5 pagesPenicillinshaitabliganNo ratings yet
- Coronary Artery DiseaseDocument38 pagesCoronary Artery Diseaseshaitabligan100% (2)
- AnthelminticsDocument8 pagesAnthelminticsshaitabliganNo ratings yet
- Anti-Malarial DrugDocument6 pagesAnti-Malarial DrugshaitabliganNo ratings yet
- Mechanisms of Action OF Antifungal AgentsDocument5 pagesMechanisms of Action OF Antifungal AgentsshaitabliganNo ratings yet
- Antibacterial CombinationsDocument21 pagesAntibacterial CombinationsshaitabliganNo ratings yet
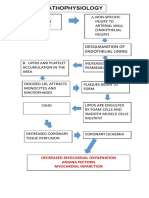
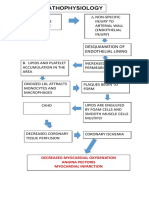
- Pathophys of CADDocument1 pagePathophys of CADshaitabliganNo ratings yet
- Pathophysiology: Risk FactorsDocument1 pagePathophysiology: Risk FactorsshaitabliganNo ratings yet
- Chronic Obstructive Pulmunary DiseaseDocument39 pagesChronic Obstructive Pulmunary DiseaseshaitabliganNo ratings yet
- Burgers DiseaseDocument21 pagesBurgers DiseaseshaitabliganNo ratings yet
- Cerebrovascular Accident: EpidemiologyDocument6 pagesCerebrovascular Accident: EpidemiologyshaitabliganNo ratings yet
- AnginaDocument4 pagesAnginashaitabliganNo ratings yet
- Myocardial InfarctionDocument28 pagesMyocardial Infarctionshaitabligan100% (1)
- Bronchial AsthmaDocument21 pagesBronchial AsthmashaitabliganNo ratings yet
- Fundamentals of Management Sample QuestionsDocument6 pagesFundamentals of Management Sample QuestionsshaitabliganNo ratings yet
- Management ProcessDocument12 pagesManagement ProcessshaitabliganNo ratings yet
- Epidemiology: Arrhythmia/DysrhythmiaDocument6 pagesEpidemiology: Arrhythmia/DysrhythmiashaitabliganNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)