Professional Documents
Culture Documents
PK, PD and Fate of Drug-1
Uploaded by
Kenny Ngowi0 ratings0% found this document useful (0 votes)
5 views59 pagesOriginal Title
PK,PD and Fate of Drug-1
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
5 views59 pagesPK, PD and Fate of Drug-1
Uploaded by
Kenny NgowiCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 59
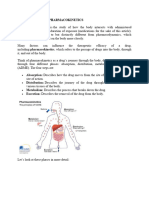
PK,PD and fate of drug.
Drug action in the body
Dr. Tosi
Definitions
• Pharmacokinetics
– The process by which a drug is absorbed,
distributed, metabolized and excreted
by the body
– Is a study of what the body does to a
drug
-In Pharmacokinetics an attempt is made to
quantify the various dispositional
parameters regarding;
• Absorption
• Distribution ADME
• Metabolism
• Excretion
ABSORPTION
• In general, for a drug to reach its intended target, the
drug must be present in the bloodstream (an
exception is application of drug for local effects such
as local anesthesia).
• Thus, absorption of drugs refers to the process
where by an amount of a drug reaches the general
circulation from its site of administration.
• The fraction of unchanged drug reaching the
systemic circulation is expressed as the
bioavailability.
Figure 2-3 Factors affecting bioavailability of drugs.
Routes of Administration
• Routes of administration greatly affect bioavailability
by changing the number of biologic barriers a drug
must cross or by changing the exposure of drug to
pumping and metabolic mechanisms.
• Enteral administration involves absorption of the drug
via the GI tract and includes oral, gastric or duodenal
(e.g., feeding tube), and rectal administration
• Parenteral administration refers to any routes of
administration that do not involve drug absorption via
the GI tract (par = around, enteral = gastrointestinal),
including the IV, intramuscular (IM), subcutaneous (SC
or SQ), and transdermal routes.
Figure 1-2 Drugs administered sublingually and rectally avoid
"first-pass metabolism" in the liver.
• The first-pass effect(first- pass metabolism or
pre systemic metabolism) is a phenomenon of
drug metabolism whereby the concentration
of a drug is greatly reduced before it reaches
the systemic circulation.
DISTRIBUTION
• After absorption into the bloodstream, drugs
are distributed to the tissues via blood flow
and diffusion and/or filtration across the
capillary membranes of various tissues.
• Initial distribution is determined by cardiac
output and regional blood flow.
– ▴ Drugs are initially distributed to tissues with the
highest blood flow (e.g., brain, lungs, kidney, and
liver).
– ▴ Tissues with lower blood flow (e.g., fat) receive
drugs later.
• Distribution to some tissue compartments is
restricted by barriers.
– ▴ The blood-brain barrier restricts distribution of
hydrophilic drugs into the brain.
Drug Redistribution
• After the initial distribution to high-blood flow
tissues, drugs redistribute to those tissues for
which they have affinity.
• Drugs may sequester in tissues for which they have
affinity.
• These tissues may then act as a sink for the drug
and increase its apparent volume of distribution
(see later).
• In addition, as plasma concentrations of drug fall,
the tissue releases drug back into the circulation,
thus prolonging the duration of action of the drug.
• In addition to specific molecular targets, drugs
show varying degrees of binding to different
components in body compartments.
• This type of binding can play an important role
in a drug's pharmacokinetic profile and in drug
interactions.
Plasma Protein Binding
• Plasma proteins, such as albumin, α-
glycoprotein, and steroid hormone binding
globulins, exhibit affinity for a number of
drugs.
• Binding to plasma protein is generally
reversible
• Plasma protein binding greatly reduces the
amount of drug free in the plasma.
• Only free drug in the plasma is able to diffuse
to its molecular site of action.
• Thus plasma protein binding can greatly
reduce the concentration of drug at the sites
of action and necessitate larger doses.
• For highly protein-bound drugs, a small change in
plasma protein binding can lead to a large change
in the proportion of free drug in the plasma and
may lead to toxicity.
– ▴ For a drug that is 99% bound to plasma protein, only
1% is free in the plasma. Reduction of plasma protein
binding to 98% results in a doubling of free drug and
drug effect.
• Changes in plasma protein binding can occur as a
result of:
– ▴ Disease
– ▴ Competition between drugs for the same binding site
– ▴ Saturation of binding sites
Tissue Binding
• Similarly to binding to plasma proteins, drugs
may also bind to individual components of
tissues.
• Binding to tissues results in sequestration of
drug in the tissue.
ELIMINATION
• Drugs are eliminated from the body via two
basic mechanisms: biotransformation
(metabolism) and excretion.
• These processes are initiated as soon as the
drug reaches the systemic circulation.
METABOLISM
• Many drugs are lipophilic molecules that
resist excretion via the kidney or gut because
they can readily diffuse back into the
circulation.
Figure 2-6 Renal mechanisms in drug excretion .
• Biotransformation is an essential step in
eliminating these drugs by converting them to
more polar water-soluble compounds.
• There are several different biotransformation
pathways that drug molecules may follow
Figure 2-5 Drug biotransformation pathways.
Biotransformation:
• May convert drugs to inactive metabolites, thus
terminating their actions
• May convert drugs to active metabolites that may have the
same or different beneficial actions as the parent drug
• May convert inactive drug molecules (prodrugs) to active
drugs
• May convert drugs to reactive intermediates that exert toxic
effects
• Occurs in many tissues including the kidney, gut, and lungs,
but for most drugs the liver is the major site of
biotransformation
• Two major processes contribute to
biotransformation of drugs.
– Phase I Reactions
– Phase II Reactions
Phase I Reactions
• Phase I reactions are also called oxidation-
reduction reactions or handle reactions.
• These reactions uncover or add a reactive
group to the drug molecule through oxidation,
reduction, or hydrolysis.
• They make the drug molecule more polar and
more reactive, which facilitates excretion or
further biotransformation of the drug through
phase II reactions.
• Oxidation accounts for a large proportion of
drug metabolism.
• It is mediated primarily by mixed function
oxygenases (monooxygenases; microsomal
mixed function oxidases) located in
endoplasmic reticulum, which include the
following:
– ▴ Cytochrome P-450 (CYP) family of enzymes
– ▴ Flavin monooxygenase family of enzymes
– ▴ Hydrolytic enzymes (e.g., epoxide hydrolase)
Phase II Reactions
• Phase II reactions are also called conjugation
reactions.
– These reactions add a polar group, such as
glucuronide, glutathione, acetate, or sulfate, to the
drug molecule.
– They increase the water solubility of compounds to
facilitate excretion.
– Generally they inactivate the drug but in some cases
can produce active metabolites (e.g., conversion of
procainamide to N-acetylprocainamide).
• Conjugation reactions can occur:
– ▴ Directly with the parent drug molecule or
– ▴ With a reactive intermediate generated by phase
I reactions.
Drug Excretion
• Drug excretion refers to the removal of drug from
the body.
• Generally, only hydrophilic molecules are
excreted effectively.
• Accordingly, drugs may be excreted as
unchanged parent molecules if they are
sufficiently hydrophilic.
• Lipophilic drugs must be biotransformed to
hydrophilic drug metabolites to be excreted.
• Drug may be excreted via a number of routes,
such as the kidney or in bile, sweat, and breast
milk.
• The lungs are an excretion route by which
volatile lipophilic substances (e.g., inhaled
general anesthetics) can be excreted.
• Changes in excretion rates will affect the
plasma concentration of drugs and their
metabolites and thus play an important role in
the design of drug regimens.
RENAL EXCRETION
• Renal excretion is quantitatively the most
important route of excretion for most drugs
and drug metabolites.
• Renal excretion involves three processes:
glomerular filtration, tubular secretion,
and/or tubular reabsorption.
• The sum of these processes determines the
extent of net renal drug excretion.
Figure 2-6 Renal mechanisms in drug excretion .
DRUG ACTION IN THE BODY
• Pharmacodynamics
– The interactions of a drug and the receptors
responsible for its action in the body
– Is a study of what the drug does to the body
• Drug molecules must be ‘bound’ to
particular constituents of cells and tissues
inorder to produce an effect
• The sites where drug molecules are bound
are called drug targets
• Most drug targets are protein molecules
• Other targets include DNA (antimicrobial
and antitumour drugs) and calcium salts
(biphosphonates)
Protein targets for drug binding
• Four main kinds of regulatory protein
commonly involved as primary drug targets
– Receptors
– Enzymes
– Carrier molecules (transporters)
– Ion channels
Drug receptors
• Definition in pharmacology: target molecule
through which soluble physiological mediators
(hormones, neurotransmitters, inflammatory
mediators, e.c.t) produce their effects.
• Other macromolecules with which drugs
interact to produce their effects are known as
drug targets
Drug specificity
• To be useful as either a therapeutic or scientific tool,
a drug must act selectively on particular cells and
tissues (show high degree of binding site specificity)
• Proteins that function as drug targets generally show
a high degree of ligand specificity – will ignore closely
related molecules
• Example: angiotensin acts strongly on vascular
smooth muscle and kidney tubules but very little
effect on other kinds of smooth muscle or intenstinal
epithellium
• However, no drug acts with complete specificity
• In general, the lower the potency of a drug, and the
higher the dose needed, the more likely is that sites
of action other than the primary one will assume
significance
Drug receptor interactions
• When drug molecule occupies a receptor, the
receptor may or may not be activated
• Receptor activation: the bound drug molecule
influences the receptor to elicit a tissue
response
• Binding and activation are two distinct steps of
the receptor-mediated tissue response
Concepts used to characterise drug
effects
• Receptor agonist: a drug which upon binding to
a receptor causes its activation
• Receptor antagonist: a drug which binds to a
receptor without causing activation thereby
preventing the agonist from binding
• Affinity of a drug: something which governs the
tendency of the drug to bind to the receptors
• Efficacy of a drug: something governing the tendency
to for the drug, once bound, to activate the receptor
• Drugs of high potency: generally have high affinity to
the receptors occupying a significant proportion of the
receptors even at low concentration
• Agonists possess high efficacy
• Antagonists have, in the simplest case, zero efficacy
• Partial agonists: drugs with intermediate level of
efficacy, even at 100 % receptor occupancy the tissue
response is submaximal
• Full agonists: drugs with sufficient efficacy that they
can elicit a maximal tissue response
• Full agonists: can produce a maximal response
(the largest response that the tissue is capable
of giving)
• Partial agonists: can produce only a
submaximal response
• The difference between full and partial
agonists lies in the relationship between
receptor occupancy and response
• The response at any given occupancy is much
smaller for the partial agonist (cannot produce
maximal response even at 100 % occupancy)
Measurement of drug effects
• Biological response measured by varying drug
concentration and observing tissue response
in an organ or tisssue bath
• This is often plotted as a concentration-effect
or dose-response curve
Uses of the dose-response curve
• Estimation of the maximal response that the
drug can produce (Emax)
• Estimation of the concentration or dose needed
to produce 50 % of maximal response (EC50 or
ED50)
• These parameters are useful in comparing
potencies of different drugs that produce
qualitatively similar effect
Figure 2-3 Experimentally observed concentration-effect curves. Although the lines, drawn according to the binding equation 2.5, fit the points well, such curves do not give correct
estimates of the affinity of drugs for receptors. This is because the relationship between receptor occupancy and response is usually non-linear.
DRUG ANTAGONISM
• Drugs interfere each other’s action
• Frequently, the effect of one drug is diminished or
completely abolished in the presence of another
• The mechanisms through which drugs do this may be
classified as follows
– Chemical antagonism
– Pharmacokinetic antagonism
– Antagonism by receptor block
– Non-competitive antagonism (block of receptor-effector
linkage)
– Physiological antagonism
Antagonism by receptor block
Physiological antagonism
Desensitisation and Tachyphylaxis
• Synonymous terms used to describe the
phenomenon whereby the effect of a drug
diminishes when it is given continously or
repeatedly.
• Desensitisation often develops within few
minutes
• Tolerance, conventionally describe a more
gradual decrease in responsiveness taking
days or weeks to develop
• Drug resistance, describe the loss of
effectiveness of antimicrobial or antitumour
drugs
• Mechanisms through which desensitasation
develops include
– Change in receptors
– Loss of receptors
– Exhaustion of mediators
– Increased metabolic degradation of the drug
– Physiological adaptation
– Active extrusion of drug from cells (mainly
relevant in cancer chemotherapy
The End!!
You might also like
- MCQs in Pharmacology PDFDocument244 pagesMCQs in Pharmacology PDFandirio748690% (10)
- Pharmadynamic - 1Document77 pagesPharmadynamic - 1Hisham AnuaimyNo ratings yet
- Pharmacology Unit 1Document58 pagesPharmacology Unit 1Asif Ali LashariNo ratings yet
- GGDocument24 pagesGGManan BhatiaNo ratings yet
- Pharmacokinetics Fate of Drugs ADMEDocument63 pagesPharmacokinetics Fate of Drugs ADMERabie YahfoufiNo ratings yet
- Pharmacology Terms & DefinitionDocument10 pagesPharmacology Terms & DefinitionKabirNo ratings yet
- Clinical PharmacologDocument81 pagesClinical PharmacologSHILOTANo ratings yet
- Module For General Zoology: Holy Cross CollegeDocument54 pagesModule For General Zoology: Holy Cross CollegeHERALD LISINGNo ratings yet
- Introduction To BiopharmaceuticsDocument106 pagesIntroduction To BiopharmaceuticsHely Patel100% (1)
- PharmacokineticsDocument34 pagesPharmacokineticsAbdelrahman GalalNo ratings yet
- Biopharmaceutics and Clinical PharmacokineticsDocument302 pagesBiopharmaceutics and Clinical PharmacokineticsBalisa MosisaNo ratings yet
- Review of Biopharmaceutics & Clinical PharmacokineticsDocument45 pagesReview of Biopharmaceutics & Clinical PharmacokineticsAmy Wu0% (1)
- Farmakokinetik Dasar At14sDocument42 pagesFarmakokinetik Dasar At14sElfiana IfaNo ratings yet
- Numerical Methods For The Life Scientist PDFDocument160 pagesNumerical Methods For The Life Scientist PDFAnonymous Zsi5ODm2PY100% (1)
- بيDocument48 pagesبيMojied AmeerNo ratings yet
- Brenner and Steven's Pharmacology 5th EditionDocument4 pagesBrenner and Steven's Pharmacology 5th Editionarief satya0% (1)
- Handbook of Drug Interaction and the Mechanism of InteractionFrom EverandHandbook of Drug Interaction and the Mechanism of InteractionRating: 1 out of 5 stars1/5 (1)
- PK, PD, Drug Interaction and Toxicology Bmls3 2020 2Document189 pagesPK, PD, Drug Interaction and Toxicology Bmls3 2020 2Kenny NgowiNo ratings yet
- Pharmacotherapeutics, Pharmacodynamics & Pharmacokinetics ExplainedDocument47 pagesPharmacotherapeutics, Pharmacodynamics & Pharmacokinetics ExplainedAshaNo ratings yet
- Pharmaco KineticsDocument42 pagesPharmaco KineticsNaghman ZuberiNo ratings yet
- Pharmacokinetics: Biological MembraneDocument18 pagesPharmacokinetics: Biological MembraneRahul PalsNo ratings yet
- Principles of Pharmacokinetics (PKDocument25 pagesPrinciples of Pharmacokinetics (PKSimonNo ratings yet
- Pharmacokinetics: Lydia B. de Castro, RNDocument37 pagesPharmacokinetics: Lydia B. de Castro, RNNick AlfaroNo ratings yet
- 3a Introduction To Drug TherapyDocument69 pages3a Introduction To Drug TherapynipoNo ratings yet
- Unit#1principle of PharmacologyDocument58 pagesUnit#1principle of PharmacologySaima VictorNo ratings yet
- Pharmacokinetics Ii: (An Idea On Drug Distribution, Metabolism and Excretion With Traditional Views.)Document49 pagesPharmacokinetics Ii: (An Idea On Drug Distribution, Metabolism and Excretion With Traditional Views.)Karthika JayanNo ratings yet
- Drug Drug Interactions Involving PsychotropsDocument72 pagesDrug Drug Interactions Involving PsychotropsSamuel FikaduNo ratings yet
- Fate of The Drug: Joanne Katherine T. Manlusoc, MSCDocument29 pagesFate of The Drug: Joanne Katherine T. Manlusoc, MSCChinenye AkwueNo ratings yet
- RUDs + PharmacokineticsDocument53 pagesRUDs + PharmacokineticsdaghameendoniaNo ratings yet
- ADME Pertemuan 2Document54 pagesADME Pertemuan 2Andi ArdiansyahNo ratings yet
- Pharmacokinetics and Drug Disposition ExplainedDocument15 pagesPharmacokinetics and Drug Disposition Explainedsachin kumarNo ratings yet
- Pharmacokinetics ADME GuideDocument81 pagesPharmacokinetics ADME GuideSiddh PatelNo ratings yet
- Cell Response 2 NotesDocument15 pagesCell Response 2 Notespranjal shahNo ratings yet
- Absorption DistributionDocument49 pagesAbsorption DistributionNorms Yoram100% (1)
- Interaksi Obat & Nutrisi 5-7-2013Document58 pagesInteraksi Obat & Nutrisi 5-7-2013Aaaniz PrastNo ratings yet
- Farmakokinetika Absorpsi, Distribusi, Metabolisme Dan EkskresiDocument47 pagesFarmakokinetika Absorpsi, Distribusi, Metabolisme Dan EkskresiRizky AfNo ratings yet
- Principle Pharmacology التمريض الادوية المحاضرة الاولى و الثانيةDocument41 pagesPrinciple Pharmacology التمريض الادوية المحاضرة الاولى و الثانيةuuuhbnb lplhghNo ratings yet
- Materi 3 - Sifat FisikokimiaDocument61 pagesMateri 3 - Sifat Fisikokimiaashley vechtersbaasNo ratings yet
- Physicochemical Properties SksDocument61 pagesPhysicochemical Properties SksGokul Raj.PNo ratings yet
- Pharma Unit 3 2022Document51 pagesPharma Unit 3 2022John Dave V. VillarmenteNo ratings yet
- PHARMACOKINETICSDocument20 pagesPHARMACOKINETICSFadri DarmaNo ratings yet
- 11-Clarke - Pharmacokinetics and TDMDocument54 pages11-Clarke - Pharmacokinetics and TDMMiski AghniaNo ratings yet
- Pharmacokinetics/ADME Pharmacokinetics/ADME in Drug DiscoveryDocument31 pagesPharmacokinetics/ADME Pharmacokinetics/ADME in Drug DiscoveryMarda Kustria DewiNo ratings yet
- Drugs and The BodyDocument84 pagesDrugs and The BodyEvelyn MedinaNo ratings yet
- Metabolic Pathway & Renal ExcretionDocument29 pagesMetabolic Pathway & Renal ExcretionRadha krishnaNo ratings yet
- 3.Unit.pharmacokinetics 2Document40 pages3.Unit.pharmacokinetics 22020253350No ratings yet
- Drug Interactions: MSC Tid 1 Nyakundi BM April 21 2010Document43 pagesDrug Interactions: MSC Tid 1 Nyakundi BM April 21 2010MONALISA MISTRINo ratings yet
- introduction (1) (1)Document56 pagesintroduction (1) (1)samrawitmekonnen16No ratings yet
- Lecture 2 - Basic Concepts of PharmacokineticsDocument63 pagesLecture 2 - Basic Concepts of Pharmacokineticsahmadslayman1No ratings yet
- Introduction to Pharmacology I (Lecture 1Document75 pagesIntroduction to Pharmacology I (Lecture 1Basil Elbushra Ahmed DomiNo ratings yet
- INTRODUCTION TO PHARMACOKINETICSDocument4 pagesINTRODUCTION TO PHARMACOKINETICSstephanienwafor18No ratings yet
- 3 PharmacokineticsDocument36 pages3 PharmacokineticsTanvir FahimNo ratings yet
- 2021-8-31 PharmacokineticsDocument25 pages2021-8-31 PharmacokineticsNOT ZUXNo ratings yet
- 01 Concept of Medication TherapyDocument145 pages01 Concept of Medication TherapytoepaNo ratings yet
- Pharma Unit 3 2022 MidtermDocument57 pagesPharma Unit 3 2022 MidtermJohn Dave V. VillarmenteNo ratings yet
- KSMU Clinical Pharmacology Principles & Rational PharmacotherapyDocument58 pagesKSMU Clinical Pharmacology Principles & Rational PharmacotherapyLucas Victor AlmeidaNo ratings yet
- Elimination: G. SrikarDocument23 pagesElimination: G. SrikarSowjanya NekuriNo ratings yet
- Therapeutic Drug Monitoring GuideDocument136 pagesTherapeutic Drug Monitoring Guidelaila kunjayatiNo ratings yet
- Pharmacokinetics: DR Narendra KumarDocument60 pagesPharmacokinetics: DR Narendra Kumarperala vinaykumarNo ratings yet
- PH CM1 Cu 3 - PharmacokineticsDocument7 pagesPH CM1 Cu 3 - Pharmacokineticseli pascualNo ratings yet
- Pharmacokinetics: "What The Body Does To The Drug"Document41 pagesPharmacokinetics: "What The Body Does To The Drug"Virgo Eri SendiNo ratings yet
- Drug MetabolisumDocument14 pagesDrug MetabolisumDevdeep Roy ChowdhuryNo ratings yet
- Drug MetabolismDocument25 pagesDrug MetabolismAbida AttaNo ratings yet
- Principle of PharmacologyDocument40 pagesPrinciple of PharmacologySolaNo ratings yet
- Drugs and BodyDocument10 pagesDrugs and BodyAlyssaGrandeMontimorNo ratings yet
- Abortions by Dr. Ng'WaliDocument29 pagesAbortions by Dr. Ng'WaliKenny NgowiNo ratings yet
- The Pharmaceuticals and Poisons Act, 9-1978 SWDocument33 pagesThe Pharmaceuticals and Poisons Act, 9-1978 SWKenny NgowiNo ratings yet
- Forensic CADocument1 pageForensic CAKenny NgowiNo ratings yet
- Food and Drug Interactions With Urine Drug Test - PPT 2Document5 pagesFood and Drug Interactions With Urine Drug Test - PPT 2Kenny NgowiNo ratings yet
- 01.0 Introduction To Forensic PathologyDocument15 pages01.0 Introduction To Forensic PathologyKenny NgowiNo ratings yet
- Schistosomiasis and Lung Flukes: Causes, Symptoms and PreventionDocument37 pagesSchistosomiasis and Lung Flukes: Causes, Symptoms and PreventionKenny NgowiNo ratings yet
- Food and Drug Interactions With Urine Drug Test - PPT 2Document5 pagesFood and Drug Interactions With Urine Drug Test - PPT 2Kenny NgowiNo ratings yet
- Ascaris LEM 2022Document36 pagesAscaris LEM 2022Kenny NgowiNo ratings yet
- AMR: Antimicrobial Resistance and MechanismsDocument40 pagesAMR: Antimicrobial Resistance and MechanismsKenny NgowiNo ratings yet
- Carter 1994Document63 pagesCarter 1994angie mendezNo ratings yet
- Oxygen Transport by HemoglobinDocument27 pagesOxygen Transport by HemoglobinLola RojasNo ratings yet
- Abstract Presentation 21428BIF007Document5 pagesAbstract Presentation 21428BIF007Divya AggarwalNo ratings yet
- Antagonist Design PDFDocument18 pagesAntagonist Design PDFLeticia AlmeidaNo ratings yet
- Ma 2011Document10 pagesMa 2011yusuf eka maulanaNo ratings yet
- (1990, Freire Et Al.) Isothermal Titration CalorimetryDocument10 pages(1990, Freire Et Al.) Isothermal Titration CalorimetryAlan R. F. K. MoraesNo ratings yet
- Glossary of Terms and Symbols Used in PharmacologyDocument55 pagesGlossary of Terms and Symbols Used in Pharmacologykriss WongNo ratings yet
- Third Generation Antipsychotic DrugsDocument45 pagesThird Generation Antipsychotic DrugsGabriela Drima100% (1)
- Biochi Ic A Et Biophysica: The Temkin Isotherm Describes Heterogeneous Protein AdsorptionDocument5 pagesBiochi Ic A Et Biophysica: The Temkin Isotherm Describes Heterogeneous Protein AdsorptionJames EdwardsNo ratings yet
- Radioligand Saturation Binding Experiments Over Large Concentration RangesDocument15 pagesRadioligand Saturation Binding Experiments Over Large Concentration Rangesrizal_pela4716No ratings yet
- 68Ga-PET: A Powerful Generator-Based Alternative To Cyclotron-Based PET RadiopharmaceuticalsDocument11 pages68Ga-PET: A Powerful Generator-Based Alternative To Cyclotron-Based PET Radiopharmaceuticalsarifudin_achmadNo ratings yet
- Beware of DockingDocument18 pagesBeware of DockingLeonardo RanderNo ratings yet
- In Silico Study of Agaricus Bisporus On DNA Damaging ProteinDocument7 pagesIn Silico Study of Agaricus Bisporus On DNA Damaging ProteinEditor IJTSRDNo ratings yet
- Antidiabetic Molecular DockingDocument19 pagesAntidiabetic Molecular DockingChristianAvelinoNo ratings yet
- Pharmaceutical ChemistryDocument21 pagesPharmaceutical ChemistryBISMA RAFIQNo ratings yet
- Theories of Drug Receptor Interactions: by Lee Eun JinDocument42 pagesTheories of Drug Receptor Interactions: by Lee Eun Jinsky.blueNo ratings yet
- E3 Ligase Ligands For PROTACsDocument19 pagesE3 Ligase Ligands For PROTACsmisganaNo ratings yet
- Meta Doc TecnicalDocument9 pagesMeta Doc TecnicalkarthidhulasiNo ratings yet
- Medicinal Chemistry: The Molecular Basis of Drug Discovery: Khan AcademyDocument17 pagesMedicinal Chemistry: The Molecular Basis of Drug Discovery: Khan AcademyRinta MoonNo ratings yet
- Protein Function: Myoglobin and HemoglobinDocument50 pagesProtein Function: Myoglobin and Hemoglobinsultan khabeebNo ratings yet
- Chapter 3 - Cell Signaling and Endocrine RegulationDocument75 pagesChapter 3 - Cell Signaling and Endocrine RegulationDee Buendia100% (1)
- Pharmacology Quizlet: Chapter 1 Intro To PharmacologyDocument18 pagesPharmacology Quizlet: Chapter 1 Intro To PharmacologyJerome ValerianoNo ratings yet
- Drug Receptor InteractionsDocument29 pagesDrug Receptor InteractionscsujithaNo ratings yet
- Rebecca L Rich and David G Myszka - Advances in Surface Plasmon Resonance Biosensor AnalysisDocument8 pagesRebecca L Rich and David G Myszka - Advances in Surface Plasmon Resonance Biosensor AnalysisKorezmNo ratings yet
- 電子全文Document274 pages電子全文張荺庭No ratings yet
- Vet Pharmacology 1 NotesDocument15 pagesVet Pharmacology 1 NotesDean Sidney LeysonNo ratings yet