Professional Documents
Culture Documents
Process Poster: Thermodynamic Processes With Their Releated Formulas
Uploaded by
AYAN MANDALOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Process Poster: Thermodynamic Processes With Their Releated Formulas
Uploaded by
AYAN MANDALCopyright:
Available Formats
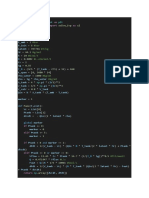
7
PROCESS
THERMODYNAMIC PROCESSES WITH THEIR RELEATED FORMULAS
POSTER
1) dW = -P
IDEA
L dv dQ + dW = Cv
2)
GAS
dT
3) dQ = Cv dT + P
dV
4)dQ = Cv dT + RT
dV/V
1)ΔU = ΔH = 0,
ISOTHERMA 2)Q = -W
L PROCESSS
3) Q =RT ln(V2/V1)
= -RT ln(P2/P1)
4) W= -RTln(V2/V1)
= RT ln(P2/P1)
ISOBARI 1) ΔU = ∫ Cv dT and
C
PROCESS 2) ΔH = ∫ Cp dT , Q =
ΔHQ = ∫ Cp
3)
dT W=-R(T2-T1)
, (Constant
P)
1) dQ = 0, dU = Cv dT =
ADIABATIC
PROCESS dWdT/T = -(R/Cv)(dV/V)
2)
3) (T2/T1) = (V1/V2)^R/Cv
4)(T2/T1) = (P1/P2)^R/Cp
5) (P1/P2) = (V1/V2)^Cp/Cv
1) ΔU = ∫ Cv dT and
ISOCHORI 2) ΔH =∫ Cp dT
C
PROCESS 3) Q = ∫ Cv dT and
4) W = - ∫ PdV=0
5) Q = ΔU = ∫ Cv
dT
ROLL NO. : 21BCHOO9
NAME : AYAN MANDAL
TUTORIAL NO.: 3
You might also like
- Formula For Engineering Thermodynamics I 254231 Semester 1 2550 Concept andDocument1 pageFormula For Engineering Thermodynamics I 254231 Semester 1 2550 Concept andKenneth MayorNo ratings yet
- Me200 - Eqnsheet 12 Jun 2012Document2 pagesMe200 - Eqnsheet 12 Jun 2012klinNo ratings yet
- THERMODYNAMICS - MODULE 2 - Lesson 4 5 - Week 8 11 - As of Nov 4Document42 pagesTHERMODYNAMICS - MODULE 2 - Lesson 4 5 - Week 8 11 - As of Nov 4Kim OpenaNo ratings yet
- TER201 Lecture 6Document66 pagesTER201 Lecture 6lnxxNo ratings yet
- 5 CHAPTER - 3b PVT EOS STDN PDFDocument44 pages5 CHAPTER - 3b PVT EOS STDN PDF许凉发100% (1)
- Ideal Gas FormulasDocument2 pagesIdeal Gas FormulasbythekiloNo ratings yet
- UntitledDocument1 pageUntitledRobert EnacheNo ratings yet
- FORMULAS XNXNDocument23 pagesFORMULAS XNXNRaymart Layson0% (1)
- Escuela Politécnica Nacional: Facultad de Ingeniería MecánicaDocument10 pagesEscuela Politécnica Nacional: Facultad de Ingeniería MecánicaAly HerreraNo ratings yet
- FORMULASDocument26 pagesFORMULASRaymart LaysonNo ratings yet
- Termodinamica ProblemDocument6 pagesTermodinamica ProblemJuandearco JuanarcoNo ratings yet
- Section A: Straight Objective Type: (Only One Correct Answer)Document12 pagesSection A: Straight Objective Type: (Only One Correct Answer)punitr2007No ratings yet
- Solution: 1. Refer Eq. (6.28)Document4 pagesSolution: 1. Refer Eq. (6.28)kajal mishrsNo ratings yet
- Sustancia Incompresible Gas Perfecto Gas Ideal Sustancia Real V U HDocument3 pagesSustancia Incompresible Gas Perfecto Gas Ideal Sustancia Real V U HOmar Jordán ÁgredaNo ratings yet
- BIS 154 - Mech Eng. 2 - Lecture 3.ppsxDocument32 pagesBIS 154 - Mech Eng. 2 - Lecture 3.ppsxMohamed NadaNo ratings yet
- 02 2ndlaw ExessolsDocument14 pages02 2ndlaw Exessolsblanca.pegueraNo ratings yet
- VL v ρV D μ: Convección Externa Forzada Convección Interna ForzadaDocument2 pagesVL v ρV D μ: Convección Externa Forzada Convección Interna ForzadaMaria JaraNo ratings yet
- Elektronički Sklopovi - Formule: U V I I I I I IDocument4 pagesElektronički Sklopovi - Formule: U V I I I I I IVanja ŽalacNo ratings yet
- Formulario Exa Final Cam FFCCDocument1 pageFormulario Exa Final Cam FFCCmalferg morriusNo ratings yet
- Physics Assignment 2 PT 2Document2 pagesPhysics Assignment 2 PT 2Mark McGarrityNo ratings yet
- Formulas P1Document1 pageFormulas P1josegandica23No ratings yet
- Exam Thermo Part1!11!12 2020 FinalDocument11 pagesExam Thermo Part1!11!12 2020 FinalMaarten ElingNo ratings yet
- Quiz 1 - MA SolutionsDocument6 pagesQuiz 1 - MA SolutionsabullaNo ratings yet
- DH Du+V DP+PDV Du+V DP H V PDocument4 pagesDH Du+V DP+PDV Du+V DP H V Pbrando1819No ratings yet
- Chapter 2 - Section A - Mathcad Solutions: M 35 KG G 9.8 M S Z 5 M Work M G Z Work 1.715 KJ U Work U 1.715 KJDocument11 pagesChapter 2 - Section A - Mathcad Solutions: M 35 KG G 9.8 M S Z 5 M Work M G Z Work 1.715 KJ U Work U 1.715 KJFaris Naufal100% (1)
- Term Odin A MicaDocument10 pagesTerm Odin A MicaFelipe De Lima RomeroNo ratings yet
- Universidad Politecnica de Madrid Escuela Técnica Superior de Ingenieros Industriales Ingeniería Del Medio AmbienteDocument6 pagesUniversidad Politecnica de Madrid Escuela Técnica Superior de Ingenieros Industriales Ingeniería Del Medio AmbienteDavid PerezNo ratings yet
- @bohring Bot × @JEE Tests 10 # GR Thermodynamics and KTG SolutionsDocument10 pages@bohring Bot × @JEE Tests 10 # GR Thermodynamics and KTG Solutionsrandomwork013No ratings yet
- Paper 1Document3 pagesPaper 1marthabervellyNo ratings yet
- Alhaji Massoud Juma - Thermo AssignmentDocument12 pagesAlhaji Massoud Juma - Thermo AssignmentAlhaj MassoudNo ratings yet
- Correction of Final January 2022Document3 pagesCorrection of Final January 2022s2ne228No ratings yet
- Formula DJJ20063Document2 pagesFormula DJJ20063NurHiday2010No ratings yet
- Control Systems Engineering D227 S.A.E. Solutions Tutorial 6 - Sinusoidal Responses Self Assessment Exercise No.1Document1 pageControl Systems Engineering D227 S.A.E. Solutions Tutorial 6 - Sinusoidal Responses Self Assessment Exercise No.1cataiceNo ratings yet
- ECE 431 Digital Circuit Design Chapter 6 MOS Inverters: Switching Characteristics and Interconnect EffectsDocument44 pagesECE 431 Digital Circuit Design Chapter 6 MOS Inverters: Switching Characteristics and Interconnect EffectsSneha S RevankarNo ratings yet
- Appendix C C. Formulae A. Chapter 1 Relations: E 2 - 14 3 2 2 2 E 24 E 6 2 C M 2 F Avog 26 26Document17 pagesAppendix C C. Formulae A. Chapter 1 Relations: E 2 - 14 3 2 2 2 E 24 E 6 2 C M 2 F Avog 26 26Ferry BudiNo ratings yet
- 4862 Solutionsxin PDFDocument37 pages4862 Solutionsxin PDFይቴ ስንሻዉNo ratings yet
- Unit 2 First Law-Closed System ProblemsDocument11 pagesUnit 2 First Law-Closed System Problemspiravi66No ratings yet
- Useful Equations For ME2121 (Part 1)Document5 pagesUseful Equations For ME2121 (Part 1)bleejunanNo ratings yet
- Chapter 3 - Section B - Non-Numerical SolutionsDocument12 pagesChapter 3 - Section B - Non-Numerical Solutionslight2618No ratings yet
- FormularioDocument1 pageFormularioLiliana GuerraNo ratings yet
- Chapter 3 - Section B - Non-Numerical SolutionsDocument10 pagesChapter 3 - Section B - Non-Numerical SolutionsFaris NaufalNo ratings yet
- AdbspDocument146 pagesAdbspKumar GauravNo ratings yet
- Chem 202 Final Exam Useful InformationDocument3 pagesChem 202 Final Exam Useful InformationNiz MuhNo ratings yet
- Physics - Solution Set For Homework Book Chapter - 15 KTG & ThermodynamicsDocument4 pagesPhysics - Solution Set For Homework Book Chapter - 15 KTG & ThermodynamicsKishorNo ratings yet
- Thermodynamic Processes: Analysis of Thermodynamic Processes by Applying 1 & 2 Law of ThermodynamicsDocument10 pagesThermodynamic Processes: Analysis of Thermodynamic Processes by Applying 1 & 2 Law of Thermodynamicsmohdmehrajanwar1860No ratings yet
- Appendix C C. Formulae A. Chapter 1 Relations: E 2 - 14 3 2 2 2 E 24 E 6 2 C M 2 F Avog 26 26Document17 pagesAppendix C C. Formulae A. Chapter 1 Relations: E 2 - 14 3 2 2 2 E 24 E 6 2 C M 2 F Avog 26 26Ivelin ValchevNo ratings yet
- CH 18Document66 pagesCH 18JuniusBrutusNo ratings yet
- P P P P: ( (P - P) / P) % 0.665 % We Can Neglect The K.E. Term in This ProblemDocument3 pagesP P P P: ( (P - P) / P) % 0.665 % We Can Neglect The K.E. Term in This ProblemAramNawzad100% (1)
- Thermodynamics Useful FormulasDocument2 pagesThermodynamics Useful FormulasKristen KellerNo ratings yet
- Internal Combustion EnginesDocument13 pagesInternal Combustion EnginesEunice Joy VillegasNo ratings yet
- OriginalDocument6 pagesOriginalyigaf49105No ratings yet
- Part Test-1 (XI)Document10 pagesPart Test-1 (XI)zoyakhan.rkeNo ratings yet
- Pemat 2Document2 pagesPemat 2Oktavianus StevianNo ratings yet
- Clase 1 - Thermodynamic OverviewDocument21 pagesClase 1 - Thermodynamic OverviewAlex Marin JimenezNo ratings yet
- Exercise II in EESDocument6 pagesExercise II in EESricardo castroNo ratings yet
- Formelsamling 2009Document18 pagesFormelsamling 2009dunderdunderNo ratings yet
- The Spectral Theory of Toeplitz Operators. (AM-99), Volume 99From EverandThe Spectral Theory of Toeplitz Operators. (AM-99), Volume 99No ratings yet