Professional Documents
Culture Documents
2 - Hess's Law
Uploaded by
Supia Nazma0 ratings0% found this document useful (0 votes)
1 views3 pagesHess's Law states that thermochemical equations can be combined algebraically to determine enthalpy changes for different chemical reactions. It provides two examples:
1) Using two equations to determine that the enthalpy change for the reaction C(s) + 1/2O2(g) → CO(g) is 110 kJ.
2) Using two equations to determine that the enthalpy change for the reaction H2O(l) → H2O(g) is 43.9 kJ.
3) It provides a third example involving the reactions of S(s) and O2(g) to form SO2(g) and SO3(g), asking
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PPT, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentHess's Law states that thermochemical equations can be combined algebraically to determine enthalpy changes for different chemical reactions. It provides two examples:
1) Using two equations to determine that the enthalpy change for the reaction C(s) + 1/2O2(g) → CO(g) is 110 kJ.
2) Using two equations to determine that the enthalpy change for the reaction H2O(l) → H2O(g) is 43.9 kJ.
3) It provides a third example involving the reactions of S(s) and O2(g) to form SO2(g) and SO3(g), asking
Copyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
1 views3 pages2 - Hess's Law
Uploaded by
Supia NazmaHess's Law states that thermochemical equations can be combined algebraically to determine enthalpy changes for different chemical reactions. It provides two examples:
1) Using two equations to determine that the enthalpy change for the reaction C(s) + 1/2O2(g) → CO(g) is 110 kJ.
2) Using two equations to determine that the enthalpy change for the reaction H2O(l) → H2O(g) is 43.9 kJ.
3) It provides a third example involving the reactions of S(s) and O2(g) to form SO2(g) and SO3(g), asking
Copyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
You are on page 1of 3
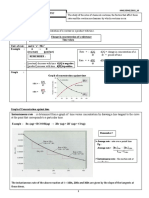
Hess’s Law
thermochemical equations can be combined
algebraically to determine enthalpy changes for a
different chemical reaction
Example 1
C(s) + O2 (g) ==> CO2(g) + 393 kJ
CO2(g) + 283 kJ ==> 1/2 O2(g) + CO(g)
-1/2 O2 and CO2(g) produces this
C(s) + 1/2 O2(g) ==> CO(g) + 110kJ
Example 2
#1 2H2O(g) + 483.2 kJ ==> 2H2(g) + O2(g)
#2 H2(g) + 1/2O2(g) ==> H2O(l) +285.5 kJ
H2O(l) +285.5 kJ ==> H2(g) + 1/2O2(g)flipped 2
H2(g) + 1/2 O2(g) ==> H2O(g) + 241.6kJ flipped 1, /2
H2O(l) + 43.9 kJ ==> H2O(g) final answer
Given the above 2 equations complete the thermo-
chemical equation for: #3 H2O(l) ==> H2O(g)
Step 12 --recognize
recognizeH H22O(g)
O(l) is a product
reactant in
(on the left)3
equation
in
andequation
in equation3. Notice
1 it is ait reactant
is found and
in equation
there are2 as a
product
2H2O(g) so so flip
flip equation
equation 2. 1 and divide it by 2.
Example 3
o
#1 S(s) + O2(g) ==> SO2(g) H = - 295.8 kJ
o
#2 S(s) + 3/2 O2(g) ==> SO3(g) H = - 394.8 kJ
SO2(g) + 295.8 kJ ==> S(s) + O2(g) flipped 1
S(s) + 3/2 O2(g) ==> SO3(g) + 394.8 kJ unchanged
SO2 (g) + 1/2 O2(g) ==>SO3(g) + 99 kJ final answer
Given the above 2 equations complete the thermo
chemical equation for: #3 SO2(g) +1/2 O2(g) =>SO3(g)
Step 21 -recognize that SO32 (g) is a product
reactant inin
equation 3 sobutleave
a product
#2 alone
in equation
but place1.theFlip
quantity
Step 3 -subtract 295.8 kJ from each side.
equation
of heat inside
1 andit.write the quantity of heat inside the
equation.
You might also like
- State - Edu/under/chemed/qbank/4/4-1/index - HTM: Changes For The Individual Steps in The Reaction."Document7 pagesState - Edu/under/chemed/qbank/4/4-1/index - HTM: Changes For The Individual Steps in The Reaction."HlajabausjNo ratings yet
- Solutions To Home Practice Test-5/Chemistry: Thermodynamics HWT - 1Document10 pagesSolutions To Home Practice Test-5/Chemistry: Thermodynamics HWT - 1varunkohliinNo ratings yet
- Hesss Law Awesome Ib Packet Questions OnlyDocument6 pagesHesss Law Awesome Ib Packet Questions OnlyEmmanuel JoyNo ratings yet
- General Chemistry Module 2 PDFDocument17 pagesGeneral Chemistry Module 2 PDFwelp100% (1)
- 5 2+Hess's+Law+ExamplesDocument4 pages5 2+Hess's+Law+ExamplesBrad Randel SolisNo ratings yet
- Hess's LawDocument7 pagesHess's Lawjax stykerNo ratings yet
- Hess S Law: Honor S Student Resource SheetDocument3 pagesHess S Law: Honor S Student Resource SheetFarz21No ratings yet
- Name - Honors Chemistry - / - / - Hess's LawDocument4 pagesName - Honors Chemistry - / - / - Hess's LawGunjee GunjeeNo ratings yet
- 5.6: Hess's Law: Learning ObjectivesDocument4 pages5.6: Hess's Law: Learning ObjectivesDhaba AberaNo ratings yet
- Experiment 2 Enthalpy of Chemical Reactions and Hess's LawDocument15 pagesExperiment 2 Enthalpy of Chemical Reactions and Hess's LawUzo Paul NwabuisiNo ratings yet
- Enthalpy and ThermochemistryDocument12 pagesEnthalpy and ThermochemistryMollin SiwellaNo ratings yet
- Prob Set 11Document3 pagesProb Set 11Payal SNo ratings yet
- 17.1 (B) Equilibrium LawDocument7 pages17.1 (B) Equilibrium LawKshiraj PanchalNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- NFTS 3.0 Thermodynamics AssignmentDocument3 pagesNFTS 3.0 Thermodynamics Assignmentvibesbb771No ratings yet
- BLB chp3Document86 pagesBLB chp3Nora Zor-elNo ratings yet
- Balancing Chemical Reactions With AnnotationsDocument39 pagesBalancing Chemical Reactions With Annotationsdyron francoNo ratings yet
- Chem 1-8Document43 pagesChem 1-8Cabacungan, John VinceNo ratings yet
- Class Work 3, PMY 221 Topic: Thermodynamics Review: For Your Calculations Take Note of The FollowingDocument2 pagesClass Work 3, PMY 221 Topic: Thermodynamics Review: For Your Calculations Take Note of The FollowingEzri ChilengweNo ratings yet
- CHEM101: General Chemistry: Chemical Reactions and Reaction StoichiometryDocument60 pagesCHEM101: General Chemistry: Chemical Reactions and Reaction Stoichiometrybarre PenroseNo ratings yet
- CHM 152 - Thermodynamics (Ch. 16) Spontaneity: False eDocument7 pagesCHM 152 - Thermodynamics (Ch. 16) Spontaneity: False eQueenQiNo ratings yet
- CEQ Ex EDocument28 pagesCEQ Ex EChess EnjoyerNo ratings yet
- Thermochemistry With SolutionsDocument4 pagesThermochemistry With SolutionsAnandhan VelayudlanNo ratings yet
- Reaction StoichiometryDocument12 pagesReaction StoichiometryAga AgaNo ratings yet
- Lesson On Gibbs' Free EnergyDocument2 pagesLesson On Gibbs' Free EnergyJan Yeasha MendezNo ratings yet
- General Chemistry Lab at Home # 2 - Writing & Balancing Chemical EquationsDocument3 pagesGeneral Chemistry Lab at Home # 2 - Writing & Balancing Chemical EquationsJayphet ChristianNo ratings yet
- Chemical FormulasDocument53 pagesChemical FormulasMARIELLE DEMINNo ratings yet
- Section - 2 Hess'S Law and Its Applications: 2 (Graphite) + 2Document31 pagesSection - 2 Hess'S Law and Its Applications: 2 (Graphite) + 2api-3728411No ratings yet
- P18 - Practice Question AnswersDocument3 pagesP18 - Practice Question AnswersdenisNo ratings yet
- CHEMICAL EQUATIONS Final VersionDocument33 pagesCHEMICAL EQUATIONS Final VersionFrancis Kirby BrutasNo ratings yet
- 1 - 1 - 1 - 1 F I Cal - 1 - 1 - 1 Cal Cal - 1 - 1 - 1 - 1 - 1Document3 pages1 - 1 - 1 - 1 F I Cal - 1 - 1 - 1 Cal Cal - 1 - 1 - 1 - 1 - 1Andrea LeopandoNo ratings yet
- Experiment 8 Sem2Document12 pagesExperiment 8 Sem2Fatin NurhudaNo ratings yet
- Lesson 3.5: Hess' Law of Heat of Summation: 2 (G) 2 (G) 2 (G) 2 (G) 2 (G) (G) (G) 2 (G) 2 (G)Document2 pagesLesson 3.5: Hess' Law of Heat of Summation: 2 (G) 2 (G) 2 (G) 2 (G) 2 (G) (G) (G) 2 (G) 2 (G)Jem SonNo ratings yet
- Topic 7. Equilibrium HL PP Pack, MarkschemeDocument17 pagesTopic 7. Equilibrium HL PP Pack, MarkschemeAylin KasaNo ratings yet
- Worksheet SpontaneityDocument3 pagesWorksheet SpontaneityElizabeth BaileyNo ratings yet
- Activity 5 Chemical Reactions and Balancing Chemical Equations IDocument6 pagesActivity 5 Chemical Reactions and Balancing Chemical Equations INivla Genesis100% (2)
- Thermochemistry PracticeDocument5 pagesThermochemistry PracticemariajoticaNo ratings yet
- Sorsogon State College: Engineering and Architecture DepartmentDocument4 pagesSorsogon State College: Engineering and Architecture DepartmentIzay Martinez CadagNo ratings yet
- Chemical ReactionDocument91 pagesChemical ReactionGlebuNo ratings yet
- Entropy-Free Energy 01 Answers PDFDocument4 pagesEntropy-Free Energy 01 Answers PDFMaddison LilyNo ratings yet
- Topic 7-17 Practice Questions Key 1 2Document8 pagesTopic 7-17 Practice Questions Key 1 2Isaline GurneNo ratings yet
- Practice Final Exam - CHEM102 - Spring 2023Document7 pagesPractice Final Exam - CHEM102 - Spring 2023mmmNo ratings yet
- EXERCISE - (JEE Main) Chemical Equilibrium - CombinedDocument24 pagesEXERCISE - (JEE Main) Chemical Equilibrium - CombinedKeerthana Reddy DomaNo ratings yet
- Thermo Test1Document3 pagesThermo Test1Aman SinghNo ratings yet
- Sorsogon State College: Engineering and Architecture DepartmentDocument4 pagesSorsogon State College: Engineering and Architecture DepartmentIzay Martinez CadagNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersFrom EverandPractice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersNo ratings yet
- Worksheet - Hess's LawDocument3 pagesWorksheet - Hess's Lawchanc49No ratings yet
- 3 - 2021 Thermodynamics USTH Part 2Document13 pages3 - 2021 Thermodynamics USTH Part 2Pham Duc AnhNo ratings yet
- Chapter 15 and 16 Revision: (104 Marks)Document26 pagesChapter 15 and 16 Revision: (104 Marks)aurennosNo ratings yet
- Exam 1 Summer08Document13 pagesExam 1 Summer08joyzevistan342No ratings yet
- Enthalpy of ReactionDocument22 pagesEnthalpy of ReactionPatricia CadacioNo ratings yet
- Module 3 - Chemical EquilibriumDocument6 pagesModule 3 - Chemical EquilibriumRuth Aquino100% (1)
- Note 9 - Chemical Equilibrium PDFDocument42 pagesNote 9 - Chemical Equilibrium PDFPamela GaudilloNo ratings yet
- ThermodynamicsDocument28 pagesThermodynamicsJack Lupino100% (2)
- Prac-2 - Balancing ReactionsDocument23 pagesPrac-2 - Balancing ReactionsCristiano PassarelliNo ratings yet
- WORK SHEET - Chemical EquilibriumDocument4 pagesWORK SHEET - Chemical EquilibriumAndrej ZafirovikjNo ratings yet
- Lesson 04: Thermochemistry Unit 02: Thermochemical Equations Learning ObjectivesDocument7 pagesLesson 04: Thermochemistry Unit 02: Thermochemical Equations Learning ObjectivesLelouchNo ratings yet
- Bab 2 Bagian ElanDocument10 pagesBab 2 Bagian ElanElan Patria NusadiNo ratings yet
- Critical Evaluation of Some Equilibrium Constants Involving Organophosphorus ExtractantsFrom EverandCritical Evaluation of Some Equilibrium Constants Involving Organophosphorus ExtractantsNo ratings yet
- Chapter 1: Matter 1.1 Atoms and Molecules: Packed in A Small NucleusDocument35 pagesChapter 1: Matter 1.1 Atoms and Molecules: Packed in A Small NucleusSupia NazmaNo ratings yet
- 2 Metallic BondsDocument13 pages2 Metallic BondsSupia NazmaNo ratings yet
- Collision Theory States That For A Reaction To OccurDocument9 pagesCollision Theory States That For A Reaction To OccurSupia NazmaNo ratings yet
- Quiz 1.1 2021 LectureDocument4 pagesQuiz 1.1 2021 LectureSupia NazmaNo ratings yet
- Quiz PHASE EQUILIBRIA (Set 3)Document4 pagesQuiz PHASE EQUILIBRIA (Set 3)Supia NazmaNo ratings yet
- Quiz PHASE EQUILIBRIA (Set 2)Document4 pagesQuiz PHASE EQUILIBRIA (Set 2)Supia NazmaNo ratings yet
- Instantaneous Rate: Is Determined From A Graph of Time Versus Concentration by Drawing A Line Tangent To The CurveDocument13 pagesInstantaneous Rate: Is Determined From A Graph of Time Versus Concentration by Drawing A Line Tangent To The CurveSupia NazmaNo ratings yet
- Exercise Born HaberDocument17 pagesExercise Born HaberSupia NazmaNo ratings yet
- Quiz C6 Set 2Document2 pagesQuiz C6 Set 2Supia NazmaNo ratings yet
- Extra Exercises - Measurement of ConcentrationDocument1 pageExtra Exercises - Measurement of ConcentrationSupia NazmaNo ratings yet
- Quiz C6 Set 4Document2 pagesQuiz C6 Set 4Supia NazmaNo ratings yet
- Quiz C5 STATES OF MATTER (Set 5)Document2 pagesQuiz C5 STATES OF MATTER (Set 5)Supia NazmaNo ratings yet
- Exercise Born HaberDocument17 pagesExercise Born HaberSupia NazmaNo ratings yet
- Quiz C6 Set 3Document1 pageQuiz C6 Set 3Supia NazmaNo ratings yet
- 19 (B)Document4 pages19 (B)Supia NazmaNo ratings yet
- Gases (B)Document115 pagesGases (B)Supia NazmaNo ratings yet
- Tutorial 1.1 (PG 1-2)Document3 pagesTutorial 1.1 (PG 1-2)Supia NazmaNo ratings yet
- Quiz C6 Set 1Document2 pagesQuiz C6 Set 1Supia NazmaNo ratings yet
- Quiz States of Matter (Set 4)Document4 pagesQuiz States of Matter (Set 4)Supia NazmaNo ratings yet
- Worksheet 1Document6 pagesWorksheet 1Supia NazmaNo ratings yet
- Gaya Komunikasi Ketua Unit (Ku) Kimia Dan Kepuasan Kerja Pensyarah Kimia Di Kolej Matrikulasi SelangorDocument12 pagesGaya Komunikasi Ketua Unit (Ku) Kimia Dan Kepuasan Kerja Pensyarah Kimia Di Kolej Matrikulasi SelangorSupia NazmaNo ratings yet
- CARBOXYLIC ACIDS Nomenclature StudentDocument23 pagesCARBOXYLIC ACIDS Nomenclature StudentSupia NazmaNo ratings yet
- Topic 14.0: Haloalkanes (Alkyl Halides)Document12 pagesTopic 14.0: Haloalkanes (Alkyl Halides)Supia NazmaNo ratings yet
- Set 1 Lampiran 1C - PelajarDocument1 pageSet 1 Lampiran 1C - PelajarSupia NazmaNo ratings yet
- Topic 14.0: Haloalkanes (Alkyl Halides)Document12 pagesTopic 14.0: Haloalkanes (Alkyl Halides)Supia NazmaNo ratings yet
- CHC NH Cooh H H CH C CH: Organic Compound That Contains Both An Amino Group, - NH2 and A Carboxyl Group, - COOHDocument6 pagesCHC NH Cooh H H CH C CH: Organic Compound That Contains Both An Amino Group, - NH2 and A Carboxyl Group, - COOHSupia NazmaNo ratings yet
- 7.0 Ionic Equilibria (Students)Document187 pages7.0 Ionic Equilibria (Students)Supia Nazma100% (1)
- Chapter 4.4-Intermolecular ForcesDocument3 pagesChapter 4.4-Intermolecular ForcesSupia NazmaNo ratings yet