0% found this document useful (0 votes)
130 views3 pagesContact Process
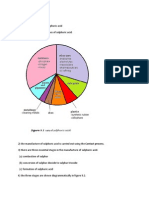
Sulphuric acid is produced using the contact process, which involves burning sulfur with oxygen to create sulfur dioxide, then converting the sulfur dioxide into sulfur trioxide with more oxygen, which is then dissolved in water to form sulphuric acid. The amount of sulphuric acid a country uses each year is indicative of its level of economic development, as it has applications in many industrial manufacturing processes like paints, detergents, fibers, plastics, and fertilizers.
Uploaded by
Kev WattsCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PPTX, PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
130 views3 pagesContact Process
Sulphuric acid is produced using the contact process, which involves burning sulfur with oxygen to create sulfur dioxide, then converting the sulfur dioxide into sulfur trioxide with more oxygen, which is then dissolved in water to form sulphuric acid. The amount of sulphuric acid a country uses each year is indicative of its level of economic development, as it has applications in many industrial manufacturing processes like paints, detergents, fibers, plastics, and fertilizers.
Uploaded by
Kev WattsCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PPTX, PDF, TXT or read online on Scribd