Professional Documents
Culture Documents
C8 Mind Map For Revision 2023
C8 Mind Map For Revision 2023
Uploaded by
emailusage0 ratings0% found this document useful (0 votes)
1 views1 pageThe document discusses factors that affect the rate of chemical reactions, including temperature, concentration, surface area, and the presence of catalysts. Higher temperatures, concentrations, and surface areas increase reaction rates by providing more opportunities for particle collisions. Catalysts also increase reaction rates by lowering the activation energy needed without being used up in the reaction.
Original Description:
Original Title
C8 Mind Map for Revision 2023
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document discusses factors that affect the rate of chemical reactions, including temperature, concentration, surface area, and the presence of catalysts. Higher temperatures, concentrations, and surface areas increase reaction rates by providing more opportunities for particle collisions. Catalysts also increase reaction rates by lowering the activation energy needed without being used up in the reaction.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
1 views1 pageC8 Mind Map For Revision 2023
C8 Mind Map For Revision 2023
Uploaded by
emailusageThe document discusses factors that affect the rate of chemical reactions, including temperature, concentration, surface area, and the presence of catalysts. Higher temperatures, concentrations, and surface areas increase reaction rates by providing more opportunities for particle collisions. Catalysts also increase reaction rates by lowering the activation energy needed without being used up in the reaction.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 1
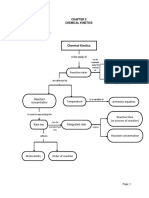
Rate of reaction is measured by Factors affecting rates of reaction:
how quickly a product is made or 1) Temperature – higher temp = faster reaction
how quickly the reactants are 2) Concentration (& pressure) – higher conc. =
used up 3 methods: faster reaction
- Decreasing mass
3) Surface Area – bigger S.A. = faster reaction
- Increasing gas product
4) Whether a catalyst is involved – catalysts speed
- Disappearing cross
up reactions
C8 – Rates of Reaction Revision
A catalyst is a substance which
Collision Theory – particles must Reversible Reactions – speeds up the rate of a reaction
collide with enough energy to reactions that can occur in both WITHOUT getting used up
cause a reaction. directions
Activation Energy = minimum HIGHER (p.122 - 125)
amount of energy needed for a Equilibrium – when the forward - Equilibrium
reaction to occur reaction is happening at the - Le Chatelier’s
same way as the backwards Principle
reaction
You might also like
- Batch and Semi-batch Reactors: Practical Guides in Chemical EngineeringFrom EverandBatch and Semi-batch Reactors: Practical Guides in Chemical EngineeringNo ratings yet
- Powerpoint - Collision Theory and Factors Affecting Rate of ReactionDocument25 pagesPowerpoint - Collision Theory and Factors Affecting Rate of ReactionAref DahabrahNo ratings yet
- Chemical Change 1Document36 pagesChemical Change 1CheloGraceTiozonAmparadoNo ratings yet
- Ap Chem Unit 5 Review PacketDocument11 pagesAp Chem Unit 5 Review Packetapi-77411869No ratings yet
- Reaction Rate NotesDocument10 pagesReaction Rate NotesvinaybharadwajbsNo ratings yet
- Topic 6 Chem NotesDocument2 pagesTopic 6 Chem NotesEmma SingerNo ratings yet
- Mind MapDocument1 pageMind MapDominic FungNo ratings yet
- Information and Communication Technology in Chemistry: Title: Collision Theory (Simulation)Document10 pagesInformation and Communication Technology in Chemistry: Title: Collision Theory (Simulation)z890% (1)
- The Collision Theory and Factors Affecting The Rate of Chemical ReactionDocument28 pagesThe Collision Theory and Factors Affecting The Rate of Chemical ReactionRolan Picones BaltazarNo ratings yet
- Rates OF ReactionDocument6 pagesRates OF ReactionAdam WilliamsNo ratings yet
- Rate of ReactionDocument6 pagesRate of ReactionDavid ThompsonNo ratings yet
- Rate of Reactions 18 April 2024Document46 pagesRate of Reactions 18 April 2024Amahle KudaNo ratings yet
- Chemistry 3 Unit 2 ReviewerDocument6 pagesChemistry 3 Unit 2 ReviewerWEEA MAE CASTRONUEVONo ratings yet
- Rates of Reaction CP2 2324Document16 pagesRates of Reaction CP2 2324estyNo ratings yet
- Collision Theory - EditedDocument17 pagesCollision Theory - EditedrhaineNo ratings yet
- Collision Theory - WJDocument16 pagesCollision Theory - WJFilzahtuln Farihah100% (1)
- IB Chemistry - Unit 6 - Chemical Kinetics Study GuideDocument6 pagesIB Chemistry - Unit 6 - Chemical Kinetics Study GuideHamzah JoharNo ratings yet
- The Rate of Chemical Reaction - NotesDocument6 pagesThe Rate of Chemical Reaction - Notesshawaiz.m.malik2010No ratings yet
- CHem InalsDocument3 pagesCHem InalsAbigail OconNo ratings yet
- Phy Sci StudyDocument2 pagesPhy Sci StudyHans Kirby Pacatang DumdumNo ratings yet
- AssignmentDocument5 pagesAssignmentAnsel MercadejasNo ratings yet
- Cheat Sheet Chemistry Chapter 6 Rate of Reaction 2Document41 pagesCheat Sheet Chemistry Chapter 6 Rate of Reaction 2yinkaNo ratings yet
- Chemical KineticsDocument13 pagesChemical KineticsAshkan SharifiyanNo ratings yet
- Chemistry Notes - VariousDocument23 pagesChemistry Notes - VariousJohn MarylandNo ratings yet
- Unit - The Periodic TableDocument20 pagesUnit - The Periodic TableMaurya AdeshraNo ratings yet
- OUTLINE - Reactions & Molecular CollisionsDocument2 pagesOUTLINE - Reactions & Molecular CollisionsJasmine Mary Issa QuezonNo ratings yet
- Chapter 6.3 BetterDocument13 pagesChapter 6.3 Betteradityayadav18julyNo ratings yet
- Reaction Rate 2024Document47 pagesReaction Rate 2024Peter KiwanukaNo ratings yet
- Reaction Rates: Aa + BB PP + QQDocument6 pagesReaction Rates: Aa + BB PP + QQtantormeNo ratings yet
- Rate of ReactionDocument23 pagesRate of ReactionVirly vcNo ratings yet
- Chapter 1-1 Rate of Chemical Reactions - StudentDocument30 pagesChapter 1-1 Rate of Chemical Reactions - StudentDhayan KomanthakkalNo ratings yet
- Rates of Reaction NotesDocument13 pagesRates of Reaction NotesNia RamNo ratings yet
- Chemisty Edexcel Unit 7 - Rates of Reaction and Energy ChangesDocument19 pagesChemisty Edexcel Unit 7 - Rates of Reaction and Energy Changesshivensolanki31No ratings yet
- 2014 Collision Theory PresentationDocument28 pages2014 Collision Theory PresentationLorato MokgethiNo ratings yet
- 8 Reaction Kinetics 23 STDDocument22 pages8 Reaction Kinetics 23 STDManh Doan DucNo ratings yet
- Chemical Kinetics PDFDocument8 pagesChemical Kinetics PDFAlexandra minNo ratings yet
- Rate of Chemical Change KODocument3 pagesRate of Chemical Change KOAyesha RahmanNo ratings yet
- Chapter 9Document18 pagesChapter 9JeromeNo ratings yet
- Factors That Affect The Rate of A Chemical ReactionDocument18 pagesFactors That Affect The Rate of A Chemical ReactionWonder Bee NzamaNo ratings yet
- Colision TheoryDocument85 pagesColision Theoryactive learning educationNo ratings yet
- Chapter 9 Rate of ReactionDocument9 pagesChapter 9 Rate of ReactionEykNo ratings yet
- Collision Theory Filzahtuln Farihah 511Document1 pageCollision Theory Filzahtuln Farihah 511Filzahtuln FarihahNo ratings yet
- AQA - A Level - Chem - 1 - Answers Ch05.inddDocument1 pageAQA - A Level - Chem - 1 - Answers Ch05.inddMahebul MazidNo ratings yet
- C8 Rates of ReactionDocument25 pagesC8 Rates of Reactionshayaanzaman0No ratings yet
- Collision TheoryDocument15 pagesCollision TheoryEfraim KasinoNo ratings yet
- Rates of ReactionDocument30 pagesRates of ReactionΜαρια ΑνδρεοπουλουNo ratings yet
- Lecture - Rate of Reaction QualitativeDocument31 pagesLecture - Rate of Reaction QualitativeWassim WsmNo ratings yet
- Module 5 Equilibrium and Acid ReactionsDocument5 pagesModule 5 Equilibrium and Acid Reactionsisaheqq12No ratings yet
- Rate of Reaction PPT 2Document11 pagesRate of Reaction PPT 2Aliyah HamiltonNo ratings yet
- Kinetics RevisionDocument3 pagesKinetics Revisionpline13579No ratings yet
- ChemistryDocument12 pagesChemistryAbdullahSohailNo ratings yet
- Chemistry 3202Document4 pagesChemistry 3202Morgan SearsNo ratings yet
- Short Note Chemistry Form 5-Chapter 1 Rate of ReactionDocument4 pagesShort Note Chemistry Form 5-Chapter 1 Rate of Reactionsalamah_sabri100% (1)
- Reaction Rates and EquilibriumDocument26 pagesReaction Rates and EquilibriumYS KWON YOUNGNo ratings yet
- Chemical Kinetics ReviewerDocument4 pagesChemical Kinetics ReviewervincentnagacNo ratings yet
- Kinetic NotesDocument1 pageKinetic NotesFrank SobelNo ratings yet
- Unit 2 Chemical Kinetics: Adnan Chowdhury Chemistry TeacherDocument9 pagesUnit 2 Chemical Kinetics: Adnan Chowdhury Chemistry TeacherZulfikarNo ratings yet
- The Following Events Must Occur Before A Reaction Can ProceedDocument33 pagesThe Following Events Must Occur Before A Reaction Can ProceedLalitha KurumanghatNo ratings yet