Professional Documents
Culture Documents
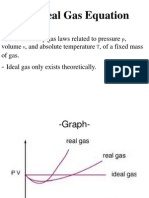
9.4 The R.M.S Speed of Molecules
Uploaded by
Noratiqah Binti Mohd Amin0 ratings0% found this document useful (0 votes)
11 views8 pageschapter 9 physics f6 1 term
Original Title
9.4 the R.M.S Speed of Molecules
Copyright
© Attribution Non-Commercial (BY-NC)
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentchapter 9 physics f6 1 term
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
11 views8 pages9.4 The R.M.S Speed of Molecules
Uploaded by
Noratiqah Binti Mohd Aminchapter 9 physics f6 1 term
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 8
9.4 The R.M.S.
Speed of Molecules
Molecules in a gas are in random motion moving in different speeds. They having different speeds due to the elastic collision between molecules. When two molecules collide elastically, kinetic energy of one molecule will transferred to other. Speed of one molecule decreases and the other increases after the collision.
r.m.s.=root-mean-square
Molecular speed distribution (pg207)
Total kinetic energy is remain constant Because the collisions are elastic When the temperature is constant, the molecular speed distribution is remains the same.
Most probable speed is the peak of the curve when the temperature of the gas is , and the speed It is the speed which the largest number of molecules have. ~Mean speed, ~Root-mean-square speed,
You might also like
- A-Level Chemistry Revision: Cheeky Revision ShortcutsFrom EverandA-Level Chemistry Revision: Cheeky Revision ShortcutsRating: 4 out of 5 stars4/5 (5)
- BI 2 - Kinetic Molecular TheoryDocument23 pagesBI 2 - Kinetic Molecular TheoryfihiNo ratings yet
- 1 Fundamental of Heat TransferDocument20 pages1 Fundamental of Heat Transferred18ggmuNo ratings yet
- Kinetic Molecular TheoryDocument8 pagesKinetic Molecular TheoryDanica Dolor PingkianNo ratings yet
- EEE 132 / ETE 132 Introduction To Materials and Chemistry Ideal Gas Equation Continued.Document13 pagesEEE 132 / ETE 132 Introduction To Materials and Chemistry Ideal Gas Equation Continued.MD. SHAEKH ZAHAB CHOWDHURYNo ratings yet
- The Kinetic Molecular Theory of MatterDocument39 pagesThe Kinetic Molecular Theory of MatterSilhouette DreamNo ratings yet
- Properties of GasesDocument12 pagesProperties of GasesshasagailNo ratings yet
- 3.1 Solids, Liquids, and GasesDocument26 pages3.1 Solids, Liquids, and GasesNaveen VangipurapuNo ratings yet
- 0 4 States of Matter-23-STDDocument26 pages0 4 States of Matter-23-STDManh Doan DucNo ratings yet
- Gas Properties and KMTDocument28 pagesGas Properties and KMTAndiNo ratings yet
- Cie Igcse Physics Chapter 2 2023 OnwDocument10 pagesCie Igcse Physics Chapter 2 2023 OnwZeinab ElkholyNo ratings yet
- Kinetic Theory of GasDocument5 pagesKinetic Theory of Gasbenazeerbeevi9No ratings yet
- Chapter 10 - States of MatterDocument31 pagesChapter 10 - States of Matterjim tannerNo ratings yet
- Kinetic-Molecular Theory: Ideal Gas Equation PV NRTDocument9 pagesKinetic-Molecular Theory: Ideal Gas Equation PV NRTVidhuPandey100% (1)
- Thermal Energy 2Document11 pagesThermal Energy 2api-276003030No ratings yet
- States of Matter: Advanced Secondary 1Document29 pagesStates of Matter: Advanced Secondary 1David JonesNo ratings yet
- Particulate Nature of MatterDocument39 pagesParticulate Nature of MatterruqwNo ratings yet
- Intermolecular Forces, Liquids and Solids: AP Chapter 11Document56 pagesIntermolecular Forces, Liquids and Solids: AP Chapter 11dNo ratings yet
- Week 1-Intermolecular Forces and Liquids and SolidsDocument19 pagesWeek 1-Intermolecular Forces and Liquids and SolidsMark John Paul CablingNo ratings yet
- NotesDocument4 pagesNotesAlaiza PandaNo ratings yet
- Conduction, Convection, & RadiationDocument14 pagesConduction, Convection, & Radiationyuvionfire100% (1)
- Chemical KineticsDocument25 pagesChemical KineticsAngelo PunzalanNo ratings yet
- Chem ReviewerDocument8 pagesChem Reviewerkaye arcedeNo ratings yet
- Day 29 - GasesDocument13 pagesDay 29 - GasesAce Vincent LunaNo ratings yet
- Pages 1 38Document38 pagesPages 1 38May Thu TheintNo ratings yet
- GASESDocument13 pagesGASESJUAN MENDOZANo ratings yet
- 4.1 Changes in The States of Matter Kinetic Theory of MatterDocument2 pages4.1 Changes in The States of Matter Kinetic Theory of MatterIbuAbi Al-FatihNo ratings yet
- Gen ChemDocument17 pagesGen Chemyousef shalabyNo ratings yet
- The Model: The Volume Occupied by The Molecules of The Gas Is Negligible Compared To The Volume of The Gas ItselfDocument1 pageThe Model: The Volume Occupied by The Molecules of The Gas Is Negligible Compared To The Volume of The Gas ItselfSameer Singh PatelNo ratings yet
- New PPTX PresentationDocument11 pagesNew PPTX PresentationMehedi HasanNo ratings yet
- Study Guide KMTDocument6 pagesStudy Guide KMT9Wezen Jowelyn Mae G. TabuzoNo ratings yet
- Teori Kenatik GasDocument12 pagesTeori Kenatik GasRoszelan Majid100% (1)
- UNIT 3 - GASES (Part 2) Gases and The Kinetik Molecular ThoeryDocument21 pagesUNIT 3 - GASES (Part 2) Gases and The Kinetik Molecular ThoeryHayden KelehanNo ratings yet
- Matter and Substances.: 4.1 Changes in The States of Matter. Kinetic Theory of MatterDocument15 pagesMatter and Substances.: 4.1 Changes in The States of Matter. Kinetic Theory of MatterElly EllynaNo ratings yet
- The Gaseous State of MatterDocument15 pagesThe Gaseous State of MatterdwijpadaliaNo ratings yet
- Kinetic TheoryDocument26 pagesKinetic TheoryDelano PeteNo ratings yet
- Physics : 5059 O Levels Methodist Girl's School Video Course Instructor: Faith Koh (A1)Document65 pagesPhysics : 5059 O Levels Methodist Girl's School Video Course Instructor: Faith Koh (A1)geoklingNo ratings yet
- Thermal PhysicsDocument47 pagesThermal Physicsjonathane nhlaneNo ratings yet
- Kinetic Molecular Model of Liquids and SolidsDocument12 pagesKinetic Molecular Model of Liquids and Solidsdavid jenil nabuaNo ratings yet
- 6.1 States of MatterDocument11 pages6.1 States of MatterHakim AbbasNo ratings yet
- Kinetic Molecular TheoryDocument22 pagesKinetic Molecular TheoryZahra NazariNo ratings yet
- Introduction To Particulate Nature of MatterDocument16 pagesIntroduction To Particulate Nature of MatterShyam BudhwarNo ratings yet
- 1.2 Kinetic Model of MatterDocument17 pages1.2 Kinetic Model of MatterHakim AbbasNo ratings yet
- Thermal Physics Brownian Motion and Absolute ZeroDocument22 pagesThermal Physics Brownian Motion and Absolute ZeroSaad BBNo ratings yet
- States of MatterDocument9 pagesStates of Mattervidya pmNo ratings yet
- Temperature and HeatDocument17 pagesTemperature and HeatABCDNo ratings yet
- Introduction HTDocument29 pagesIntroduction HTGoutam VijNo ratings yet
- Molar Specific Heats of Other MaterialsDocument25 pagesMolar Specific Heats of Other MaterialsShootingStarPhotonsNo ratings yet
- 1.the Particulate Nature of MatterDocument25 pages1.the Particulate Nature of MatterEdward DhlaminiNo ratings yet
- 1.the Particulate Nature of MatterDocument25 pages1.the Particulate Nature of MatterEdwardNo ratings yet
- Notes Kinetic Molecular TheoryDocument4 pagesNotes Kinetic Molecular TheoryGino Carlos MiguelNo ratings yet
- Chapter 4 States of Matter AS LEVEL NOTESDocument6 pagesChapter 4 States of Matter AS LEVEL NOTESArslnNo ratings yet
- Chapter 8pt2Document23 pagesChapter 8pt2Stephen Rey CaldeaNo ratings yet
- EN1101 - MJE - Part 9 - Phase Diagrams - LCDocument17 pagesEN1101 - MJE - Part 9 - Phase Diagrams - LCnwankwo chubyNo ratings yet
- Liquids and SolidsDocument14 pagesLiquids and SolidsKurt Jan PlopenioNo ratings yet
- Kinetic Model of MatterDocument75 pagesKinetic Model of MatterDELVIN CARRIENo ratings yet
- Kinetic Molecular TheoryDocument19 pagesKinetic Molecular TheoryMariane HinanibanNo ratings yet
- Temperature and HeatDocument17 pagesTemperature and HeatYMANUELLE THERESE LEIGH LAZARITONo ratings yet
- Temperature and Heat: Heat Is A Flow of Energy Due To Temperature DifferencesDocument17 pagesTemperature and Heat: Heat Is A Flow of Energy Due To Temperature DifferencesDEVAUGHN ANTIFUESTONo ratings yet
- Reinforcement Exercise Chapter 3Document3 pagesReinforcement Exercise Chapter 3Noratiqah Binti Mohd AminNo ratings yet
- Reinforcement 11.05Document2 pagesReinforcement 11.05Noratiqah Binti Mohd AminNo ratings yet
- Group 8 4.1 Thermal EquilbriumDocument16 pagesGroup 8 4.1 Thermal EquilbriumNoratiqah Binti Mohd AminNo ratings yet
- 4.2 Specific Heat CapacityDocument25 pages4.2 Specific Heat CapacityNoratiqah Binti Mohd AminNo ratings yet
- Physics LessonDocument2 pagesPhysics LessonNoratiqah Binti Mohd AminNo ratings yet
- Totally Reflecting Prisms: Total Internal Reflection MirrorDocument3 pagesTotally Reflecting Prisms: Total Internal Reflection MirrorNoratiqah Binti Mohd AminNo ratings yet
- Chapter 4 - Fluid Mechanics - Fis - 2020Document69 pagesChapter 4 - Fluid Mechanics - Fis - 2020Noratiqah Binti Mohd AminNo ratings yet
- Teks Pengacara Majlis Sekalung Budi Sejambak Kasih - LaguDocument1 pageTeks Pengacara Majlis Sekalung Budi Sejambak Kasih - LaguNoratiqah Binti Mohd AminNo ratings yet
- TEKS PENGACARA MAJLIS SEKALUNG BUDI SEJAMBAK KASIH - LaguDocument1 pageTEKS PENGACARA MAJLIS SEKALUNG BUDI SEJAMBAK KASIH - LaguNoratiqah Binti Mohd AminNo ratings yet
- Ytp Hon Phy F5Document4 pagesYtp Hon Phy F5Noratiqah Binti Mohd AminNo ratings yet
- Teks Pengacara Majlis Sekalung Budi Sejambak Kasih - LaguDocument1 pageTeks Pengacara Majlis Sekalung Budi Sejambak Kasih - LaguNoratiqah Binti Mohd AminNo ratings yet
- The Assessment Framework of Physics Project 960/4 STPM 2019/2020 Sekolah Menengah JK Chung Hwa, Kelantan Assessment Project ReportDocument1 pageThe Assessment Framework of Physics Project 960/4 STPM 2019/2020 Sekolah Menengah JK Chung Hwa, Kelantan Assessment Project ReportNoratiqah Binti Mohd AminNo ratings yet
- The Assessment Framework of Physics Project 960/4 STPM 2019/2020 Sekolah Menengah JK Chung Hwa, Kelantan Assessment Project ReportDocument1 pageThe Assessment Framework of Physics Project 960/4 STPM 2019/2020 Sekolah Menengah JK Chung Hwa, Kelantan Assessment Project ReportNoratiqah Binti Mohd AminNo ratings yet
- Analisis Fizik STPM Updated 2018 ADocument11 pagesAnalisis Fizik STPM Updated 2018 ANoratiqah Binti Mohd AminNo ratings yet
- 9.5 Degree of Freedom and Law of EquipartitionDocument6 pages9.5 Degree of Freedom and Law of EquipartitionNoratiqah Binti Mohd AminNo ratings yet
- SPM Fizik Tingkatan 4,5 - Paper2 - 20120724090124Document24 pagesSPM Fizik Tingkatan 4,5 - Paper2 - 20120724090124Noratiqah Binti Mohd AminNo ratings yet
- Silibus f6Document132 pagesSilibus f6Noratiqah Binti Mohd AminNo ratings yet
- Rekod PDPC (Noratiqah) : Bulatan Buih Buih Berganda Pokok Dakap Alir Pelbagai Alir Titi Share Your View Idea RushDocument3 pagesRekod PDPC (Noratiqah) : Bulatan Buih Buih Berganda Pokok Dakap Alir Pelbagai Alir Titi Share Your View Idea RushNoratiqah Binti Mohd AminNo ratings yet
- CheckingDocument1 pageCheckingNoratiqah Binti Mohd AminNo ratings yet
- It Is A RealityDocument1 pageIt Is A RealityNoratiqah Binti Mohd AminNo ratings yet
- 9.5 Degree of Freedom and Law of EquipartitionDocument6 pages9.5 Degree of Freedom and Law of EquipartitionNoratiqah Binti Mohd AminNo ratings yet
- Skrip Pengacara Majlis Asean English VersionDocument5 pagesSkrip Pengacara Majlis Asean English VersionNoratiqah Binti Mohd Amin100% (3)
- Physics 9.1Document6 pagesPhysics 9.1Noratiqah Binti Mohd AminNo ratings yet