Professional Documents
Culture Documents
Naming Flowchart
Naming Flowchart
Uploaded by
stephaniey101Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Naming Flowchart
Naming Flowchart
Uploaded by
stephaniey101Copyright:
Available Formats
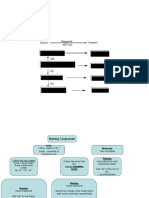
Flow Chart for Naming Simple Inorganic Compounds The flowchart is adapted from p.
131-132 of the February 1983 issue of the Journal of Chemical Education. Does the formula begin with H? No Yes Are there two atoms, both the same? Yes No It is an acid. Does the acid contain a polyatomic ion? (More than two elements.) Yes No
It is the diatomic gas hydrogen. Does it begin with a metal which has more than one oxidation number? Fe, Ni, Cu, Sn, Hg, Pb, Co, Cr, Au No Yes Does the polyatomic end in -ate or -ite? -ate -ite Name the first element followed by its oxidation number. (Roman numeral) Name the polyatomic ion, replacing -ate with -ic. Add the word acid. Name the polyatomic ion, replacing -ite with -ous. Add the word acid. Write the prefix hydro, then the name of the second element with the -ic ending. Add the word acid.
Does the formula contain a polyatomic ion? (More than two elements.) No Yes
Is the polyatomic written first? No Yes
Name the first element, then the polyatomic ion. (If two elements are present, name both, then the polyatomic ion.) Are both elements nonmetals? No Yes
Name the polyatomic first, then name the element second. If there are two polyatomics, name the first, then the second.
Name the first element, then the second element with -ide ending.
Are both elements the same? No Yes It is a diatomic element. The compound has the same name as the element.
Name the first element using the proper prefix (di, tri, etc.), but never mono. Name the second element with the proper prefix (including mono) and the -ide ending.
You might also like
- Chemistry Naming Compounds Flow ChartDocument1 pageChemistry Naming Compounds Flow ChartBob SmithNo ratings yet
- Naming Compounds Flow ChartDocument2 pagesNaming Compounds Flow ChartKeith JosephsNo ratings yet
- Naming CompoundsDocument10 pagesNaming CompoundsIan Miala MorenoNo ratings yet
- Jhoan Rhea L. Pizon, PHDDocument59 pagesJhoan Rhea L. Pizon, PHDRancell Vincent Gablines MorenoNo ratings yet
- Naming Compounds 1Document32 pagesNaming Compounds 1MARILOU SARANo ratings yet
- Naming Compounds 1Document32 pagesNaming Compounds 1kristineNo ratings yet
- Flowchart For Naming Ionic CompoundsDocument1 pageFlowchart For Naming Ionic Compoundsapi-246864303No ratings yet
- Naming Flowchart ChemistryDocument1 pageNaming Flowchart ChemistryamyNo ratings yet
- Atomic Structures Formulas and NamesDocument4 pagesAtomic Structures Formulas and NamesKyla Mari ValduezaNo ratings yet
- Module On Ions, Molecules and CompoundsDocument6 pagesModule On Ions, Molecules and CompoundsRelox, Kenth Gabriel R.No ratings yet
- Nomenclature FlowchartDocument2 pagesNomenclature FlowchartaresamritNo ratings yet
- 2genchem PreMidterm NotesDocument13 pages2genchem PreMidterm NoteshjNo ratings yet
- The Modern View of Atomic Structure: An Introduction 2.5-2.8Document3 pagesThe Modern View of Atomic Structure: An Introduction 2.5-2.8Anita LopesNo ratings yet
- Lesson 4: Naming Ions and Compounds and Deriving Chemical FormulasDocument13 pagesLesson 4: Naming Ions and Compounds and Deriving Chemical FormulasAljon CatibanNo ratings yet
- Naming CompoundsDocument17 pagesNaming CompoundsTrisha Mae ArellanoNo ratings yet
- Chemistry: Quarter 1 - Module 7: "Naming Compounds"Document12 pagesChemistry: Quarter 1 - Module 7: "Naming Compounds"NormanNo ratings yet
- AtomsDocument5 pagesAtomskrisha mandriqueNo ratings yet
- 2 - Atomic StructureDocument2 pages2 - Atomic StructureAugene BoncalesNo ratings yet
- General Chemistry: Chapter Ii I. Chemical Formulas and Nomenclature (Naming of Compounds)Document6 pagesGeneral Chemistry: Chapter Ii I. Chemical Formulas and Nomenclature (Naming of Compounds)Celive SiendaNo ratings yet
- Inorganic Nomenclature Worksheet 2Document7 pagesInorganic Nomenclature Worksheet 2Ji-Shawn PardassieNo ratings yet
- Reviewer in ChemistryDocument11 pagesReviewer in Chemistryxian tanNo ratings yet
- GenChem1 3rd Quarter ReviewerDocument3 pagesGenChem1 3rd Quarter ReviewerkathreenezyanaNo ratings yet
- Binary CompoundsDocument1 pageBinary CompoundsMarlon Mata Barkada LangNo ratings yet
- GenChem1 Q1 Lesson-7Document45 pagesGenChem1 Q1 Lesson-7jr LalemNo ratings yet
- Detailed Lesson Plan in Science 8: P - OT - N N - TRO - N - LE - TR - NDocument8 pagesDetailed Lesson Plan in Science 8: P - OT - N N - TRO - N - LE - TR - NMc Laurence Marquez SaligumbaNo ratings yet
- L2 Atoms, Molecules and IonsDocument5 pagesL2 Atoms, Molecules and IonsJohn Mark Clouie PlacaNo ratings yet
- 02 Chemical NomenclatureDocument4 pages02 Chemical Nomenclatureronnel.feloniaNo ratings yet
- Formulas of CompoundsDocument2 pagesFormulas of CompoundsMarcus MatanguihanNo ratings yet
- Chapter TwoDocument47 pagesChapter Twoeliasferhan1992No ratings yet
- Chemical Changes - Knowledge OrganiserDocument2 pagesChemical Changes - Knowledge OrganiserMai Hương NguyễnNo ratings yet
- Naming Chemical CompoundsDocument41 pagesNaming Chemical CompoundsroviannmaehlidemNo ratings yet
- CH 7 NotesDocument5 pagesCH 7 NotesAnand PandeyNo ratings yet
- Naming Compounds FlowchartDocument1 pageNaming Compounds Flowchartapi-310503032No ratings yet
- "FLOW CHART" Use When Naming Compounds!: Is It A Transition Metal?Document1 page"FLOW CHART" Use When Naming Compounds!: Is It A Transition Metal?Angelica Yamilex BocanegraNo ratings yet
- Laws of Matter and Daltons Atomic TheoryDocument17 pagesLaws of Matter and Daltons Atomic Theoryjerome deiparineNo ratings yet
- Laws of Matter and Daltons Atomic TheoryDocument17 pagesLaws of Matter and Daltons Atomic TheoryPrince SanjiNo ratings yet
- Naming CompoundsDocument16 pagesNaming Compoundsapi-268606328No ratings yet
- ATOMS MOLECULES Notes Shri Radhe 24 1664985666851Document12 pagesATOMS MOLECULES Notes Shri Radhe 24 1664985666851Divyansh RanaNo ratings yet
- Le - Co1Document2 pagesLe - Co1Aiko FloresNo ratings yet
- Chapter 2.2Document7 pagesChapter 2.2Exelsis LeanoNo ratings yet
- Naming CompoundsDocument60 pagesNaming CompoundsLorilieNo ratings yet
- Subject Terms CHDocument3 pagesSubject Terms CHPalak SwainNo ratings yet
- Ionic or MolecularDocument2 pagesIonic or MolecularBrandy JessicaNo ratings yet
- Subject Terms Chemistry UseDocument3 pagesSubject Terms Chemistry UsePalak SwainNo ratings yet
- Naming Compounds and Formula WritingDocument5 pagesNaming Compounds and Formula WritingNicoleNo ratings yet
- Revision-Map Chapter 3Document1 pageRevision-Map Chapter 3Megha BishtNo ratings yet
- Chemistry Material PropertiesDocument17 pagesChemistry Material PropertiesMaulana Digisel DesainNo ratings yet
- (F A.aon: at N"utonDocument2 pages(F A.aon: at N"utonmisstsangNo ratings yet
- Lesson 2.5 Chemical NomenclatureDocument6 pagesLesson 2.5 Chemical NomenclaturerejymolNo ratings yet
- Atoms, Molecules, and IonsDocument36 pagesAtoms, Molecules, and IonsTherese ArellanoNo ratings yet
- Chapter 2 - 2.6 Compounds and FormulaeDocument9 pagesChapter 2 - 2.6 Compounds and FormulaeDeepak KumarNo ratings yet
- Naming Inorganic CompoundsDocument29 pagesNaming Inorganic CompoundsLove TakaNo ratings yet
- GENCHEM1 12 Q1 Week3 Mod7 MELC07 MOD Aurellano JesusDocument17 pagesGENCHEM1 12 Q1 Week3 Mod7 MELC07 MOD Aurellano Jesuswencylle casilNo ratings yet
- Atom Molecule: L L L L LDocument1 pageAtom Molecule: L L L L LJitendra KumarNo ratings yet
- General Chemistry - DocsDocument17 pagesGeneral Chemistry - DocsJohn leeNo ratings yet
- Naming Chemical Compounds: Rules and TipsDocument5 pagesNaming Chemical Compounds: Rules and TipsNoah G.No ratings yet
- Periodic Table TestDocument5 pagesPeriodic Table TestRica RoscoNo ratings yet
- Why Are Chemicals Not Named John? Naming Chemical Compounds 6th Grade | Children's Chemistry BooksFrom EverandWhy Are Chemicals Not Named John? Naming Chemical Compounds 6th Grade | Children's Chemistry BooksNo ratings yet
- How Is Mercury Used Today? Chemistry Book for Kids 9-12 | Children's Chemistry BooksFrom EverandHow Is Mercury Used Today? Chemistry Book for Kids 9-12 | Children's Chemistry BooksNo ratings yet
- (CHEM) Pairwork Chem NomencDocument1 page(CHEM) Pairwork Chem NomencsodiumboyupinthishoeNo ratings yet
- (CHEM) Chemical ReactionsDocument32 pages(CHEM) Chemical Reactionssodiumboyupinthishoe100% (2)
- Chemical-Bonding & Inter Molecular ForcesDocument6 pagesChemical-Bonding & Inter Molecular ForcesLMT_GORDON100% (1)
- Rules For Assigning An Oxidation NumberDocument1 pageRules For Assigning An Oxidation NumbernegrabelleNo ratings yet
- Animal Tissues 1Document8 pagesAnimal Tissues 1Valen IgnacioNo ratings yet
- (BIO) 03 - Animal Tissues (Calsado)Document12 pages(BIO) 03 - Animal Tissues (Calsado)sodiumboyupinthishoeNo ratings yet
- Electronics Experimenters Handbook 1993Document132 pagesElectronics Experimenters Handbook 1993Benjamin Dover100% (2)
- To Estimate Protein Content in Unknown Sample Using Bradford's Assay.Document3 pagesTo Estimate Protein Content in Unknown Sample Using Bradford's Assay.John kNo ratings yet
- Lectuer 8Document7 pagesLectuer 8Chandra MynNo ratings yet
- SikaPlast-518 (Ro) SDS - ENGDocument10 pagesSikaPlast-518 (Ro) SDS - ENGmuzafferkeskin2020No ratings yet
- Self-Emulsifying Drug Delivery Systems (SEDDS) For Improved Oral Delivery of Lipophilic DrugsDocument10 pagesSelf-Emulsifying Drug Delivery Systems (SEDDS) For Improved Oral Delivery of Lipophilic DrugsAna KovačevićNo ratings yet
- RVT TowerPackings EN WEB 20220804Document5 pagesRVT TowerPackings EN WEB 20220804Göksel VATANNo ratings yet
- Air Blast Circuit BreakerDocument16 pagesAir Blast Circuit BreakerKhalid QureshiNo ratings yet
- The Effects of A K SO Solution On The Surface Hardness of Gypsum Type IIIDocument7 pagesThe Effects of A K SO Solution On The Surface Hardness of Gypsum Type IIIYuvraj KapoorNo ratings yet
- Radioactive Mind MapDocument16 pagesRadioactive Mind Mapwahidms840% (1)
- Pollution Control in Fertilizer and PetroleumDocument24 pagesPollution Control in Fertilizer and PetroleumCheriyan EbenezerNo ratings yet
- Page 1 of 5 Name: Section: Lecture 13. THERMOCHEMISTRY: Prepared By: Philip B. Pacot JR., Special Science Teacher 1Document5 pagesPage 1 of 5 Name: Section: Lecture 13. THERMOCHEMISTRY: Prepared By: Philip B. Pacot JR., Special Science Teacher 1Typical PiaNo ratings yet
- Who Par Guidance With AppendixesDocument69 pagesWho Par Guidance With AppendixeslanikhilNo ratings yet
- Y10May21 HWDocument8 pagesY10May21 HWMahmud RahmanNo ratings yet
- (2018) Effects of Magnesium Oxide On Carbonic Acid Resistance of Oil Well CementDocument13 pages(2018) Effects of Magnesium Oxide On Carbonic Acid Resistance of Oil Well CementRamón RamalhoNo ratings yet
- Unit V-Production Cost Estimation: Session 1 RecapDocument34 pagesUnit V-Production Cost Estimation: Session 1 RecapvengadeshNo ratings yet
- 11 Macadam Asphalt MatDocument41 pages11 Macadam Asphalt MatLovely Mae Cruza GawinganNo ratings yet
- WW Microbiology PresentationDocument35 pagesWW Microbiology PresentationAudrius100% (1)
- T CrioDocument2 pagesT CrioTempcoNo ratings yet
- iCE 3000 Series Detection LimitsDocument1 pageiCE 3000 Series Detection LimitsdudutwaeNo ratings yet
- Registered Pesticide October 2005 - January 2012Document255 pagesRegistered Pesticide October 2005 - January 2012dev26v80% (1)
- Advanced Gas Turbine Cycles For Power GenerationDocument10 pagesAdvanced Gas Turbine Cycles For Power GenerationRenzo Alexander RestrepoNo ratings yet
- MOLYKOTE P-37 Paste 80-3092C-01Document2 pagesMOLYKOTE P-37 Paste 80-3092C-01murat OZKANNo ratings yet
- R FR B1Document39 pagesR FR B1risnasilvi13No ratings yet
- New Simplified Chemistry ClassDocument11 pagesNew Simplified Chemistry ClassbabuNo ratings yet
- 2022L - Revest de Resistência À Erosão Por Cavitação Usando Aço Inox e Ligas de Cobalto Depositados Por Gmaw e CW GmawDocument14 pages2022L - Revest de Resistência À Erosão Por Cavitação Usando Aço Inox e Ligas de Cobalto Depositados Por Gmaw e CW GmawIgor Alexsander Barbosa MagnoNo ratings yet
- Characterization of Polymeric Foams PDFDocument35 pagesCharacterization of Polymeric Foams PDFDenisse JiménezNo ratings yet
- Stainless Steel Grades 2008 01Document2 pagesStainless Steel Grades 2008 01Rahul LavandNo ratings yet
- CHEM F313: Instrumental Methods of Analysis: UV-Vis Spectrometry (Contd.)Document17 pagesCHEM F313: Instrumental Methods of Analysis: UV-Vis Spectrometry (Contd.)AYUSH SHARMANo ratings yet
- Bohler Colour ChartDocument1 pageBohler Colour Chartscooba84No ratings yet
- 811 4x3-13a40 - PERFDocument20 pages811 4x3-13a40 - PERFCARLOSFERDEMAN234PONo ratings yet