Professional Documents
Culture Documents
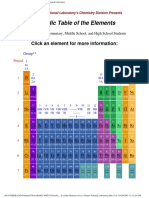
IUPAC Periodic Table of The Elements: Ti CR
Uploaded by
Marcus LimaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
IUPAC Periodic Table of The Elements: Ti CR
Uploaded by
Marcus LimaCopyright:
Available Formats
1
H
hydrogen
[1.007; 1.009]
1 18
3
Li
lithium
[6.938; 6.997]
4
Be
beryllium
9.012
11
Na
sodium
22.99
12
Mg
magnesium
24.31
19
K
potassium
39.10
20
Ca
calcium
40.08
37
Rb
rubidium
85.47
38
Sr
strontium
87.62
38
Sr
strontium
87.62
55
Cs
caesium
132.9
55
Cs
caesium
132.9
56
Ba
barium
137.3
87
Fr
francium
88
Ra
radium
5
B
boron
[10.80; 10.83]
13
Al
aluminium
26.98
31
Ga
gallium
69.72
49
In
indium
114.8
81
Tl
thallium
[204.3; 204.4]
6
C
carbon
[12.00; 12.02]
14
Si
silicon
[28.08; 28.09]
32
Ge
germanium
72.63
50
Sn
tin
118.7
82
Pb
lead
207.2
7
N
nitrogen
[14.00; 14.01]
15
P
phosphorus
30.97
33
As
arsenic
74.92
51
Sb
antimony
121.8
83
Bi
bismuth
209.0
8
O
oxygen
[15.99; 16.00]
16
S
sulfur
[32.05; 32.08]
34
Se
selenium
78.96(3)
52
Te
tellurium
127.6
84
Po
polonium
9
F
fluorine
19.00
17
Cl
chlorine
[35.44; 35.46]
35
Br
bromine
79.90
53
I
iodine
126.9
85
At
astatine
10
Ne
neon
20.18
2
He
helium
4.003
18
Ar
argon
39.95
36
Kr
krypton
83.80
54
Xe
xenon
131.3
86
Rn
radon
22
Ti
titanium
47.87
22
Ti
titanium
47.87
40
Zr
zirconium
91.22
72
Hf
hafnium
178.5
104
Rf
rutherfordium
23
V
vanadium
50.94
41
Nb
niobium
92.91
73
Ta
tantalum
180.9
105
Db
dubnium
24
Cr
chromium
52.00
24
Cr
chromium
52.00
42
Mo
molybdenum
95.96(2)
74
W
tungsten
183.8
106
Sg
seaborgium
25
Mn
manganese
54.94
43
Tc
technetium
75
Re
rhenium
186.2
107
Bh
bohrium
26
Fe
iron
55.85
44
Ru
ruthenium
101.1
76
Os
osmium
190.2
108
Hs
hassium
27
Co
cobalt
58.93
45
Rh
rhodium
102.9
77
Ir
iridium
192.2
109
Mt
meitnerium
28
Ni
nickel
58.69
46
Pd
palladium
106.4
78
Pt
platinum
195.1
110
Ds
darmstadtium
29
Cu
copper
63.55
47
Ag
silver
107.9
79
Au
gold
197.0
30
Zn
zinc
65.38(2)
48
Cd
cadmium
112.4
80
Hg
mercury
200.6
111
Rg
roentgenium
112
Cn
copernicium
114
Fl
flerovium
116
Lv
livermorium
57
La
lanthanum
138.9
89
Ac
actinium
58
Ce
cerium
140.1
90
Th
thorium
232.0
59
Pr
praseodymium
140.9
91
Pa
protactinium
231.0
60
Nd
neodymium
144.2
92
U
uranium
238.0
61
Pm
promethium
93
Np
neptunium
62
Sm
samarium
150.4
94
Pu
plutonium
63
Eu
europium
152.0
95
Am
americium
64
Gd
gadolinium
157.3
96
Cm
curium
65
Tb
terbium
158.9
97
Bk
berkelium
66
Dy
dysprosium
162.5
98
Cf
californium
67
Ho
holmium
164.9
99
Es
einsteinium
68
Er
erbium
167.3
100
Fm
fermium
69
Tm
thulium
168.9
101
Md
mendelevium
70
Yb
ytterbium
173.1
102
No
nobelium
71
Lu
lutetium
175.0
103
Lr
lawrencium
21
Sc
scandium
44.96
39
Y
yttrium
88.91
57-71
lanthanoids
89-103
actinoids
atomic number
Symbol
standard atomic weight
2 13 14 15 16 17 Key:
3 4 5 6 7 8 9 10 11 12
name
Notes
- IUPAC 2009 Standard atomic weights abridged to four signicant digits (Table 4 published in Pure Appl. Chem. 83, 359-396 (2011);
doi:10.1351/PAC-REP-10-09-14). The uncertainty in the last digit of the standard atomic weight value is listed in parentheses following the value.
In the absence of parentheses, the uncertainty is one in that last digit. An interval in square brackets provides the lower and upper bounds of the
standard atomic weight for that element. No values are listed for elements which lack isotopes with a characteristic isotopic abundance in natural
terrestrial samples. See PAC for more details.
- Aluminum and cesium are commonly used alternative spellings for aluminium and caesium.
- Claims for the discovery of all the remaining elements in the last row of the Table, namely elements with atomic numbers 113, 115, 117 and 118,
and for which no assignments have yet been made, are being considered by a IUPAC and IUPAP Joint Working Party.
For updates to this table, see iupac.org/reports/periodic_table/. This version is dated 1 June 2012.
Copyright 2012 IUPAC, the International Union of Pure and Applied Chemistry.
IUPAC Periodic Table of the Elements
INTERNATIONAL UNION OF
PURE AND APPLIED CHEMISTRY
1
H
hydrogen
[1.007; 1.009]
1 18
3
Li
lithium
[6.938; 6.997]
4
Be
beryllium
9.012
11
Na
sodium
22.99
12
Mg
magnesium
24.31
19
K
potassium
39.10
20
Ca
calcium
40.08
37
Rb
rubidium
85.47
38
Sr
strontium
87.62
38
Sr
strontium
87.62
55
Cs
caesium
132.9
55
Cs
caesium
132.9
56
Ba
barium
137.3
87
Fr
francium
88
Ra
radium
5
B
boron
[10.80; 10.83]
13
Al
aluminium
26.98
31
Ga
gallium
69.72
49
In
indium
114.8
81
Tl
thallium
[204.3; 204.4]
6
C
carbon
[12.00; 12.02]
14
Si
silicon
[28.08; 28.09]
32
Ge
germanium
72.63
50
Sn
tin
118.7
82
Pb
lead
207.2
7
N
nitrogen
[14.00; 14.01]
15
P
phosphorus
30.97
33
As
arsenic
74.92
51
Sb
antimony
121.8
83
Bi
bismuth
209.0
8
O
oxygen
[15.99; 16.00]
16
S
sulfur
[32.05; 32.08]
34
Se
selenium
78.96(3)
52
Te
tellurium
127.6
84
Po
polonium
9
F
fluorine
19.00
17
Cl
chlorine
[35.44; 35.46]
35
Br
bromine
79.90
53
I
iodine
126.9
85
At
astatine
10
Ne
neon
20.18
2
He
helium
4.003
18
Ar
argon
39.95
36
Kr
krypton
83.80
54
Xe
xenon
131.3
86
Rn
radon
22
Ti
titanium
47.87
22
Ti
titanium
47.87
40
Zr
zirconium
91.22
72
Hf
hafnium
178.5
104
Rf
rutherfordium
23
V
vanadium
50.94
41
Nb
niobium
92.91
73
Ta
tantalum
180.9
105
Db
dubnium
24
Cr
chromium
52.00
24
Cr
chromium
52.00
42
Mo
molybdenum
95.96(2)
74
W
tungsten
183.8
106
Sg
seaborgium
25
Mn
manganese
54.94
43
Tc
technetium
75
Re
rhenium
186.2
107
Bh
bohrium
26
Fe
iron
55.85
44
Ru
ruthenium
101.1
76
Os
osmium
190.2
108
Hs
hassium
27
Co
cobalt
58.93
45
Rh
rhodium
102.9
77
Ir
iridium
192.2
109
Mt
meitnerium
28
Ni
nickel
58.69
46
Pd
palladium
106.4
78
Pt
platinum
195.1
110
Ds
darmstadtium
29
Cu
copper
63.55
47
Ag
silver
107.9
79
Au
gold
197.0
30
Zn
zinc
65.38(2)
48
Cd
cadmium
112.4
80
Hg
mercury
200.6
111
Rg
roentgenium
112
Cn
copernicium
114
Fl
flerovium
116
Lv
livermorium
57
La
lanthanum
138.9
89
Ac
actinium
58
Ce
cerium
140.1
90
Th
thorium
232.0
59
Pr
praseodymium
140.9
91
Pa
protactinium
231.0
60
Nd
neodymium
144.2
92
U
uranium
238.0
61
Pm
promethium
93
Np
neptunium
62
Sm
samarium
150.4
94
Pu
plutonium
63
Eu
europium
152.0
95
Am
americium
64
Gd
gadolinium
157.3
96
Cm
curium
65
Tb
terbium
158.9
97
Bk
berkelium
66
Dy
dysprosium
162.5
98
Cf
californium
67
Ho
holmium
164.9
99
Es
einsteinium
68
Er
erbium
167.3
100
Fm
fermium
69
Tm
thulium
168.9
101
Md
mendelevium
70
Yb
ytterbium
173.1
102
No
nobelium
71
Lu
lutetium
175.0
103
Lr
lawrencium
21
Sc
scandium
44.96
39
Y
yttrium
88.91
57-71
lanthanoids
89-103
actinoids
atomic number
Symbol
standard atomic weight
2 13 14 15 16 17 Key:
3 4 5 6 7 8 9 10 11 12
name
Notes
- IUPAC 2009 Standard atomic weights abridged to four signicant digits (Table 4 published in Pure Appl. Chem. 83, 359-396 (2011);
doi:10.1351/PAC-REP-10-09-14). The uncertainty in the last digit of the standard atomic weight value is listed in parentheses following the value.
In the absence of parentheses, the uncertainty is one in that last digit. An interval in square brackets provides the lower and upper bounds of the
standard atomic weight for that element. No values are listed for elements which lack isotopes with a characteristic isotopic abundance in natural
terrestrial samples. See PAC for more details.
- Aluminum and cesium are commonly used alternative spellings for aluminium and caesium.
- Claims for the discovery of all the remaining elements in the last row of the Table, namely elements with atomic numbers 113, 115, 117 and 118,
and for which no assignments have yet been made, are being considered by a IUPAC and IUPAP Joint Working Party.
For updates to this table, see iupac.org/reports/periodic_table/. This version is dated 1 June 2012.
Copyright 2012 IUPAC, the International Union of Pure and Applied Chemistry.
IUPAC Periodic Table of the Elements
INTERNATIONAL UNION OF
PURE AND APPLIED CHEMISTRY
You might also like
- Periodic TableDocument1 pagePeriodic Tablebudi_alamsyahNo ratings yet
- Student Pocket HandbookDocument64 pagesStudent Pocket Handbookadarsh_mrNo ratings yet
- Heat and Mass Transfer Yunus A Cengel AppendixDocument27 pagesHeat and Mass Transfer Yunus A Cengel Appendixafiq_akashah1264No ratings yet
- Honors Chemistry WKSHT Periodic Table IA ANSWERSDocument10 pagesHonors Chemistry WKSHT Periodic Table IA ANSWERSKaleb HuttoNo ratings yet
- Table of MetalsDocument26 pagesTable of MetalsAkramNo ratings yet
- David Besser - Cambridge IGCSE™ Chemistry Study and Revision Guide Third Edition-Hodder EducationDocument172 pagesDavid Besser - Cambridge IGCSE™ Chemistry Study and Revision Guide Third Edition-Hodder EducationSuha DawNo ratings yet
- Materials Data for Cyclic Loading: Aluminium and Titanium AlloysFrom EverandMaterials Data for Cyclic Loading: Aluminium and Titanium AlloysRating: 1 out of 5 stars1/5 (1)
- Periodic Table Packet WorksheetDocument5 pagesPeriodic Table Packet WorksheetNiña Mariz PacilanNo ratings yet
- ANSWERS - Yr 10 Chem Practice Test QuestionsDocument8 pagesANSWERS - Yr 10 Chem Practice Test QuestionsJerryNo ratings yet
- Phys Sci GR 10 Summaries, Terms, Definitions, Activities 9 April 2020Document206 pagesPhys Sci GR 10 Summaries, Terms, Definitions, Activities 9 April 2020Tasmiyah Kader100% (1)
- ATOMIC WEIGHTS OF THE ELEMENTS 2013Document8 pagesATOMIC WEIGHTS OF THE ELEMENTS 2013akvssakthivelNo ratings yet
- Periodic TableDocument1 pagePeriodic TableAshok LakshmananNo ratings yet
- AlloysDocument27 pagesAlloysranvir_rajNo ratings yet
- AiCHe Student Pocket Handbook 85Document63 pagesAiCHe Student Pocket Handbook 85DigitalMastersTXNo ratings yet
- Elements - Metals, Non-Metals and Semi-MetalsDocument4 pagesElements - Metals, Non-Metals and Semi-MetalsLulu Li100% (1)
- Linear Coefficients of ExpansionDocument12 pagesLinear Coefficients of ExpansionVBT1No ratings yet
- CULLITY, B. STORCK, S. Elements of X-Ray Diffraction. 3. Ed. - Ap. 13. Crystal Structure Data (P. 488-491) PDFDocument4 pagesCULLITY, B. STORCK, S. Elements of X-Ray Diffraction. 3. Ed. - Ap. 13. Crystal Structure Data (P. 488-491) PDFFernando Freitas AlvesNo ratings yet
- Periodic TableDocument133 pagesPeriodic TableKailasam MNo ratings yet
- 3.3 The Periodic TableDocument19 pages3.3 The Periodic TableKislay GaurNo ratings yet
- Periodic Table Overview: Elements, Groups & TrendsDocument169 pagesPeriodic Table Overview: Elements, Groups & TrendsMalik DaniyalNo ratings yet
- Periodic table elements in Chinese charactersDocument3 pagesPeriodic table elements in Chinese charactersTheodore HaralabisNo ratings yet
- C. R. Brazier Et Al - Laser Spectroscopy of Alkaline Earth Monoalkoxide Free RadicalsDocument7 pagesC. R. Brazier Et Al - Laser Spectroscopy of Alkaline Earth Monoalkoxide Free RadicalsLupaessNo ratings yet
- Periodic TableDocument137 pagesPeriodic TableIshfaqAhmedMayoNo ratings yet
- VOGEL Química Analítica Qualitativa 7ed PDFDocument356 pagesVOGEL Química Analítica Qualitativa 7ed PDFMiguel Machado Manhães100% (1)
- Compuestos Cationicos de Aluminio Con Relevamcia Potecial para La Catalisis Del Ácido de LewisDocument2 pagesCompuestos Cationicos de Aluminio Con Relevamcia Potecial para La Catalisis Del Ácido de LewisALEJANDRA JIMENEZNo ratings yet
- Aqa Chm6x W Ins Jun11Document2 pagesAqa Chm6x W Ins Jun11Illharm SherrifNo ratings yet
- Platinum-Gold Nanoparticles: A Highly Active Bifunctional Electrocatalyst For Rechargeable Lithium-Air BatteriesDocument4 pagesPlatinum-Gold Nanoparticles: A Highly Active Bifunctional Electrocatalyst For Rechargeable Lithium-Air BatteriesAnthony RussellNo ratings yet
- Electronic Supplementary InformationDocument11 pagesElectronic Supplementary InformationLOLA PATRICIA MORALES DE LA CUBANo ratings yet
- Organometallic Compounds: 1952 by The Recognition ofDocument9 pagesOrganometallic Compounds: 1952 by The Recognition oflaythNo ratings yet
- 1992 Lazaridis Daf Metal IonsDocument16 pages1992 Lazaridis Daf Metal IonsAhmed AliNo ratings yet
- CESL Copper Arsenic StabilityDocument12 pagesCESL Copper Arsenic Stabilitysonia gutierezNo ratings yet
- Metallurgy Research PaperDocument2 pagesMetallurgy Research Papervirotix812No ratings yet
- Comparision of The Flow in Co-Rotating and Counter-Rotating TwinscrewDocument11 pagesComparision of The Flow in Co-Rotating and Counter-Rotating TwinscrewChauNo ratings yet
- A Periodic Table of The Elements at Los Alamos National LaboratoryDocument3 pagesA Periodic Table of The Elements at Los Alamos National Laboratoryروشان فاطمة روشانNo ratings yet
- Atomic Weights IUPACDocument17 pagesAtomic Weights IUPACHHSpapayaNo ratings yet
- Chem Data BookletDocument48 pagesChem Data Bookletnikf_6No ratings yet
- Phase Transformations in Metals and Alloys, Porter y Easterling (2ed.) BC-305-331Document13 pagesPhase Transformations in Metals and Alloys, Porter y Easterling (2ed.) BC-305-331NIKOLEE LIZETH TORRES ZUÑIGANo ratings yet
- CBC Databook 1Document36 pagesCBC Databook 1anees19oct50% (2)
- PERIODIC TABLE ELEMENTSDocument7 pagesPERIODIC TABLE ELEMENTSOtgon OrgilNo ratings yet
- Study Guide - AnswersDocument8 pagesStudy Guide - Answerssophieee marieeeNo ratings yet
- Chapter SixDocument33 pagesChapter SixasifNo ratings yet
- Periodic Table of The ElementsDocument2 pagesPeriodic Table of The ElementsReeja MathewNo ratings yet
- Aturan PaulingDocument39 pagesAturan PaulingMeyga Evi Ferama SariNo ratings yet
- Synthesis and Structure Rhodium Complexes Containing A Photolabile Q - Carbodiimlde Ligand. 1,3-Dipolar Cycloaddition of Phenyl Azide To TP'RH (CNR) P (TP' H Ydrotris (3,5-Dimethylpyrazolyi) Borate)Document10 pagesSynthesis and Structure Rhodium Complexes Containing A Photolabile Q - Carbodiimlde Ligand. 1,3-Dipolar Cycloaddition of Phenyl Azide To TP'RH (CNR) P (TP' H Ydrotris (3,5-Dimethylpyrazolyi) Borate)Nguyễn Thanh TùngNo ratings yet
- Ex4 Mocox-F03Document8 pagesEx4 Mocox-F03Anonymous cgKtuWzNo ratings yet
- Anie 201500570 PDFDocument6 pagesAnie 201500570 PDFSiddiqui M. M.No ratings yet
- 2019 Atomic WeightsDocument7 pages2019 Atomic WeightsMirella PopescuNo ratings yet
- Investigation of The Electron Spin Resonance Spectra of Van 1978 Journal ofDocument4 pagesInvestigation of The Electron Spin Resonance Spectra of Van 1978 Journal ofFihad LatheefNo ratings yet
- A Simulation Study of AlkanesDocument11 pagesA Simulation Study of AlkanesLucasSuffrediniNo ratings yet
- (CHEM) Order Among The ElementsDocument54 pages(CHEM) Order Among The ElementsJeanneNo ratings yet
- Periodictable BWDocument1 pagePeriodictable BWShubham SinghNo ratings yet
- IB Chemistry Data Book 2009Document48 pagesIB Chemistry Data Book 2009phantomdancerNo ratings yet
- Acid Pressure Oxidation of ArsenopyriteDocument8 pagesAcid Pressure Oxidation of ArsenopyriteEdgar PérezNo ratings yet
- Good Colour Periodic TableDocument1 pageGood Colour Periodic TableDaizLee AhmadNo ratings yet
- Dictionary of Chemical Formulas - Wikipedia, The Free EncyclopediaDocument60 pagesDictionary of Chemical Formulas - Wikipedia, The Free EncyclopediaNoorNo ratings yet
- NSS Chemistry Part 3 Metals - LQDocument25 pagesNSS Chemistry Part 3 Metals - LQNicole ChanNo ratings yet
- Periodic Table IsotopesDocument1 pagePeriodic Table IsotopeslamantreveurNo ratings yet
- Coordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972From EverandCoordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972A. B. P. LeverNo ratings yet
- Experimental and Theoretical Approaches to Actinide ChemistryFrom EverandExperimental and Theoretical Approaches to Actinide ChemistryJohn K. GibsonNo ratings yet
- Application of IC-MS and IC-ICP-MS in Environmental ResearchFrom EverandApplication of IC-MS and IC-ICP-MS in Environmental ResearchRajmund MichalskiNo ratings yet
- Vibrational Spectra of Organometallics: Theoretical and Experimental DataFrom EverandVibrational Spectra of Organometallics: Theoretical and Experimental DataNo ratings yet
- Cifra Club - ANGEL - AugustanaDocument3 pagesCifra Club - ANGEL - AugustanaMarcus LimaNo ratings yet
- Imprimir - Cifra Club - WHAT's UP - 4 Non BlondesDocument4 pagesImprimir - Cifra Club - WHAT's UP - 4 Non BlondesMarcus LimaNo ratings yet
- How You Remind Me Chords... e at Ultimate-GuitarDocument2 pagesHow You Remind Me Chords... e at Ultimate-GuitarMarcus LimaNo ratings yet
- The Light Before We LandDocument2 pagesThe Light Before We LandMarcus LimaNo ratings yet
- Electron Configuration WoDocument3 pagesElectron Configuration WoClaude CaduceusNo ratings yet
- Lesson 1 in Organic Chemistry (MBS 524)Document29 pagesLesson 1 in Organic Chemistry (MBS 524)id.villegas.sciencenorthNo ratings yet
- Atomic & Molecular StructureDocument233 pagesAtomic & Molecular StructureeihdqdlmNo ratings yet
- Con Review WKSHTDocument216 pagesCon Review WKSHTgkapsNo ratings yet
- Chemistry The Molecular Nature of Matter and Change 7th Edition Silberberg Solutions ManualDocument19 pagesChemistry The Molecular Nature of Matter and Change 7th Edition Silberberg Solutions Manualshute.scasely.i94b100% (23)
- Tajuk Fokus Penting Kimia SPM 2013Document4 pagesTajuk Fokus Penting Kimia SPM 2013sukichokiNo ratings yet
- Periodic Table Part 1 HandoutDocument8 pagesPeriodic Table Part 1 HandoutChristopher Jr TundagNo ratings yet
- The Periodic Table Reading ComprehensionDocument4 pagesThe Periodic Table Reading ComprehensionRayyanNo ratings yet
- EAMCET-QR-Chemistry-Jr Chem-2.Classification of Elements and Periodicity in PropertiesDocument13 pagesEAMCET-QR-Chemistry-Jr Chem-2.Classification of Elements and Periodicity in PropertiespvnchemNo ratings yet
- Science 2019 Sa2 SolutionsDocument14 pagesScience 2019 Sa2 SolutionsNeha malavNo ratings yet
- Science Practicum Periodic Table Lesson PlanDocument3 pagesScience Practicum Periodic Table Lesson Planapi-341413691100% (1)
- GRADE 8 3rd & 4thDocument4 pagesGRADE 8 3rd & 4thVenus BusalpaNo ratings yet
- Early Models of Periodic TableDocument2 pagesEarly Models of Periodic TableOm TipsetwarNo ratings yet
- List of Chemical ElementsDocument2 pagesList of Chemical ElementsJhon Vincent Draug Posadas100% (1)
- CHAPTER # 1the Nature of Matter and Periodicity of Atomic PropertiesDocument48 pagesCHAPTER # 1the Nature of Matter and Periodicity of Atomic PropertiesAnonymous UfzcLV8ZNo ratings yet
- Grade 10 Science (Chemistry Unit)Document12 pagesGrade 10 Science (Chemistry Unit)Aastha SinghNo ratings yet
- Group IV Chemistry-1Document18 pagesGroup IV Chemistry-1SEBAGGALA YUNUSNo ratings yet
- The Halogen FamilyDocument21 pagesThe Halogen FamilyAshish KumarNo ratings yet
- Hl.12.1 Electrons in AtomsDocument31 pagesHl.12.1 Electrons in AtomsJerry LouNo ratings yet
- The Periodic Table Its Story and Its SignificanceDocument3 pagesThe Periodic Table Its Story and Its SignificanceJason Vinluan CarinanNo ratings yet
- Periodicity Quick RecapDocument8 pagesPeriodicity Quick RecapApeksha MudagiNo ratings yet
- Elements by Atomic MassDocument4 pagesElements by Atomic MassHaider AliNo ratings yet
- Chemistry Handout (Part I)Document64 pagesChemistry Handout (Part I)Thiha MyoNo ratings yet
- Scientific Method Experiments ApplesDocument47 pagesScientific Method Experiments ApplesitsmesinghNo ratings yet
- Periodic Table Basics - Answer KeyDocument3 pagesPeriodic Table Basics - Answer KeyJunk0% (1)
- EEE 221: Physical Electronics: Engr. Dr. H. O. Ohize April 28, 2021Document24 pagesEEE 221: Physical Electronics: Engr. Dr. H. O. Ohize April 28, 2021Quantum BoyNo ratings yet
- Chapter 8 Periodic TableDocument9 pagesChapter 8 Periodic TablenothingisfingnothingNo ratings yet