Professional Documents
Culture Documents
Quantum
Uploaded by
farzad1556Original Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Quantum
Uploaded by
farzad1556Copyright:
Available Formats
Farzad Ramesh MM111054
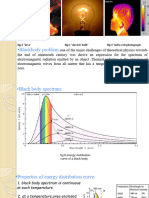
the attempts to express the spectrum of the electromagnetic energy emitted by an object in thermal
equilibrium at a temperature T, called black body was resulted to weins formula for the spectrum of the
black bodys radiation:
S(, I) = o
3
c
-[] 1
That only worked when the frequency was high. Another formula came from classical physics for low
frequencies known as the Rayleigh-Jeans formula:
S(, I) =
8n
2
c
3
k
B
I
Where k
B
Boltzmanns constant. But there were some flows; it says that an object at any temperature
would radiate an infinite amount of energy at infinity high frequencies.
To solve this problem Planck assumed black body absorbed and emitted light in multiples quantum of
energy E = , = 6.6218 1u
-34
S(, I) =
8n
3
c
3
1
c
(
h]
kt
-1)
Einstein believed light of angular frequency was made of packets of energy quanta which could be
only absorbed and emitted completely.
Bohr considered a Hydrogen atom consisted of a positive and a negative charged particles .classical
electromagnetism says when the electron accelerates; it radiates on a time scale of ~ 1u
-12
scc.it passes
a spiral way into the proton meanwhile and finally the atom destructs itself.
To solve this problem Bohr defined the quantization condition. According to that, electron circulates in a
specific radius that satisfies the formula: I = m:r = n
Where l is the angular momentum, v the speed of electron, m the mass and n is the quantum number. In
this theory the orbits are stable and the energies that the atom can have are:
E
n
=
kc
2
2o
0
1
n
2
, k =
1
4ne
0
, o
0
=
4ne
0
2
mc
2
= u.uS29 nm
The frequency of the photon emitted in a transition from quantum number ni to the number nf would be:
=
E
n
E
n]
=
kc
2
2o
0
_
1
n
2
]
1
n
2
_
Einstein used these ideas of Bohr to rederive the black body spectrum result of Planck. He also expressed
spontaneous emission and found out that light quanta is more than packets of energy. But carry
momentum in a particular direction.
You might also like
- Elementary Particles: The Commonwealth and International LibraryFrom EverandElementary Particles: The Commonwealth and International LibraryNo ratings yet
- Blackbody Radiation PDFDocument24 pagesBlackbody Radiation PDFAtul SinghNo ratings yet
- Lecture 2Document10 pagesLecture 2Ifra NoorNo ratings yet
- Che101 Chap 7Document47 pagesChe101 Chap 7David MaranzhyanNo ratings yet
- Quantum Mechanics PyEd 342Document113 pagesQuantum Mechanics PyEd 342mesfint100% (3)
- Quantum Field Theory RadiationDocument5 pagesQuantum Field Theory RadiationieiediediedieNo ratings yet
- Wave Mechanics and The SCHR Odinger Equation: 1.1 Historical Foundations of Quantum PhysicsDocument9 pagesWave Mechanics and The SCHR Odinger Equation: 1.1 Historical Foundations of Quantum PhysicsPhyo ThihaNo ratings yet
- Electronic Structure of AtomsDocument59 pagesElectronic Structure of Atomstalktotiffanycheng100% (1)
- Che101 Chap 7Document48 pagesChe101 Chap 7Ruhi AfsaraNo ratings yet
- Ch1 Revision of EP203Document31 pagesCh1 Revision of EP203mirckyNo ratings yet
- Quantum MechanicsDocument38 pagesQuantum MechanicsSoumyadeep DasNo ratings yet
- EMP Lec 1,2Document8 pagesEMP Lec 1,2Prem MurjaniNo ratings yet
- CHEM-UA 127: Advanced General Chemistry I: I. The Classical View of The UniverseDocument10 pagesCHEM-UA 127: Advanced General Chemistry I: I. The Classical View of The Universehkalloli@gmail.comNo ratings yet
- Blackbody RadiationDocument23 pagesBlackbody RadiationkowletNo ratings yet
- Unit 11 Modern PhysicsDocument90 pagesUnit 11 Modern PhysicsPeril LousNo ratings yet
- Physics HSCDocument2 pagesPhysics HSCmothermonkNo ratings yet
- Física Cuántica SencillaDocument35 pagesFísica Cuántica SencillaSamuel Carrillo100% (1)
- QuantumDocument388 pagesQuantumyana33No ratings yet
- Early HistoryDocument12 pagesEarly HistoryShweta SridharNo ratings yet
- 8-Ch7 (Struktur Atom)Document91 pages8-Ch7 (Struktur Atom)Mia YukimuraNo ratings yet
- Engineering Physics Lecture Notes Module 1Document36 pagesEngineering Physics Lecture Notes Module 1Damodhar reddy GarlapatiNo ratings yet
- Very Brief Introduction To Quantum MechanicsDocument66 pagesVery Brief Introduction To Quantum Mechanicspav163No ratings yet
- Inorganic Chemistry 1semesterDocument12 pagesInorganic Chemistry 1semestermanjunathu731No ratings yet
- Chem Bonding - Sukanta - Class LectureDocument85 pagesChem Bonding - Sukanta - Class LecturePavan Kethavath100% (1)
- Quantum NoteDocument30 pagesQuantum NoteSenevirathne K.M.M.C.No ratings yet
- General Chemistry Notes 3Document32 pagesGeneral Chemistry Notes 3Robert P.No ratings yet
- QM BasicsDocument12 pagesQM Basicsh.anurag248No ratings yet
- Inorganic Chemistry I-VIDocument134 pagesInorganic Chemistry I-VIMurad AlDamen100% (3)
- Chapter 6 Jan13Document131 pagesChapter 6 Jan13kumutha100% (1)
- 5) Quantum Mechanics - UpdatedDocument86 pages5) Quantum Mechanics - UpdatedGame 1No ratings yet
- Che 402 e Photochemistry NotesDocument20 pagesChe 402 e Photochemistry NotesKEVIN OMONDINo ratings yet
- Max Planck and Black Body RadiationDocument3 pagesMax Planck and Black Body RadiationMarie Joy EngayNo ratings yet
- Wave Mechanics LectureDocument94 pagesWave Mechanics LectureJunnoKaiserNo ratings yet
- AP Physics Chapter 27 Quantum PhysicsDocument78 pagesAP Physics Chapter 27 Quantum PhysicsMythos EntertainmentNo ratings yet
- CH 2 The Particle Properties of WavesDocument21 pagesCH 2 The Particle Properties of Wavesyohanse mehabawNo ratings yet
- Black Body RadiationDocument33 pagesBlack Body RadiationSafnas Kariapper100% (1)
- Electronic Structure of Atoms (2023)Document66 pagesElectronic Structure of Atoms (2023)Tshegofatso DipaleNo ratings yet
- 4) Quantum MechanicsDocument75 pages4) Quantum MechanicsGame 1No ratings yet
- 1237.quantum Mechanics 1Document94 pages1237.quantum Mechanics 1cattlNo ratings yet
- Blackbody RadiationDocument9 pagesBlackbody RadiationTitin Evania ManaluNo ratings yet
- CY1101 - Atomic STR - Usha PDFDocument73 pagesCY1101 - Atomic STR - Usha PDFChiranjit DasNo ratings yet
- OriginDocument52 pagesOriginRajat PatodiNo ratings yet
- Photon Concept and It'S Application By: Jaidee CastroDocument16 pagesPhoton Concept and It'S Application By: Jaidee CastroBA N TOT SU ME RANo ratings yet
- The Strange World of Quantum Mechanics: and BeautifulDocument24 pagesThe Strange World of Quantum Mechanics: and Beautifulgshubham7No ratings yet
- 2nd MeetDocument48 pages2nd MeetIntan CahyaningrumNo ratings yet
- MIT8 04S16 LecNotes3 PDFDocument8 pagesMIT8 04S16 LecNotes3 PDFJefersonNo ratings yet
- Atomic Theory 2Document5 pagesAtomic Theory 2Husen HasenNo ratings yet
- Quan Mech and Chemical KineticsDocument37 pagesQuan Mech and Chemical KineticsKevin Rick SantosNo ratings yet
- Quantum PDFDocument388 pagesQuantum PDFJelena RadivojevicNo ratings yet
- PPT1 and 2Document43 pagesPPT1 and 2Adugnaw BiksNo ratings yet
- Quantum MechanicsDocument6 pagesQuantum MechanicsRINA MORENONo ratings yet
- History of Quantum MechanicsDocument22 pagesHistory of Quantum MechanicsLinh ChiNo ratings yet
- PlanckDocument15 pagesPlanckPrincess ZoyaNo ratings yet
- Wave Model - Hydrogen Case - 2023-2024Document36 pagesWave Model - Hydrogen Case - 2023-2024rahmaderradji23No ratings yet
- Quantum PhysicsDocument122 pagesQuantum PhysicsYang Xu100% (2)
- Waves o ParticlesDocument6 pagesWaves o ParticlesFlor Ruiz VelaNo ratings yet
- Assignment: BS PhysicsDocument10 pagesAssignment: BS PhysicsRehmanNo ratings yet
- Early and Modern Q1Document2 pagesEarly and Modern Q1Prabhat RayNo ratings yet
- Mahasiswa Mampu Menjelaskan Konsep Kuantisasi Energi: Kompetensi 1Document43 pagesMahasiswa Mampu Menjelaskan Konsep Kuantisasi Energi: Kompetensi 1Imam SyNo ratings yet
- TuanAnh Chapter 1 AtomsDocument64 pagesTuanAnh Chapter 1 AtomsTrần Gia LinhNo ratings yet
- Jackson 4 1 Homework SolutionDocument6 pagesJackson 4 1 Homework SolutionRafaela MedeirosNo ratings yet
- Chapter ThreeDocument21 pagesChapter ThreeProf. Dr. Hassan N. Al-ObaidiNo ratings yet
- Algebras and Their Covariant Representations in Quantum GravityDocument21 pagesAlgebras and Their Covariant Representations in Quantum Gravitykdl1187056915No ratings yet
- BSC Part IiiDocument70 pagesBSC Part IiiInvincible HeroNo ratings yet
- Application of 1st Order de in Drainage of A Water TankDocument5 pagesApplication of 1st Order de in Drainage of A Water TankIrsyad Sony100% (1)
- QMsol18 3 PDFDocument25 pagesQMsol18 3 PDFRitaNo ratings yet
- Natural System of Units in General RelativityDocument5 pagesNatural System of Units in General Relativitymukesh2194No ratings yet
- CBSE Class 12 Maths Question Paper Solution 2020 Set 65 5 1Document10 pagesCBSE Class 12 Maths Question Paper Solution 2020 Set 65 5 1AjiteshNo ratings yet
- 11 - Rotations in Ordinary Space PDFDocument17 pages11 - Rotations in Ordinary Space PDFUltrazordNo ratings yet
- Sec 4 Revision 6 - Probability and VectorsDocument1 pageSec 4 Revision 6 - Probability and Vectorswill bNo ratings yet
- Observer Design For Discrete-Time Singular Systems With Time-Varying DelayDocument6 pagesObserver Design For Discrete-Time Singular Systems With Time-Varying Delayghassen marouaniNo ratings yet
- Plasticity TheoryDocument540 pagesPlasticity TheoryMukesh Muthu100% (2)
- مبدأ عدم اليقينDocument3 pagesمبدأ عدم اليقينSara AliNo ratings yet
- Dualisme Cahaya Sebagai Gelombang Dan Partikel: Wave Properties Light IntensityDocument31 pagesDualisme Cahaya Sebagai Gelombang Dan Partikel: Wave Properties Light IntensityTitis AndriawanNo ratings yet
- What This Module Is About: Force and MotionDocument10 pagesWhat This Module Is About: Force and MotionCeasar Epsilonian MundalaNo ratings yet
- PhysicsDocument17 pagesPhysicsJomaina AhmedNo ratings yet
- An Approach, To Understanding Psychotronics PDFDocument89 pagesAn Approach, To Understanding Psychotronics PDFLeonard Loch100% (1)
- PlanimeterDocument4 pagesPlanimeterdukenmargaNo ratings yet
- Spin Orbit CouplingDocument8 pagesSpin Orbit CouplingRajdeep BanerjeeNo ratings yet
- UCLA Physics Articulation AgreementDocument2 pagesUCLA Physics Articulation AgreementkensusantoNo ratings yet
- ED of K.P ModelDocument7 pagesED of K.P ModelShareefMondaNo ratings yet
- Oxford Master Course in Mathematical and Theoretical PhysicsDocument67 pagesOxford Master Course in Mathematical and Theoretical PhysicsH LNo ratings yet
- Ladrsm2e Páginas 43 44Document2 pagesLadrsm2e Páginas 43 44Nicolás Camargo RojasNo ratings yet
- Math P4 Past Paper QuestionsDocument17 pagesMath P4 Past Paper QuestionsShubangi PareshNo ratings yet
- Maxwells EquationsDocument45 pagesMaxwells EquationsArif JemaliNo ratings yet
- 16.20 - Structural Mechanics Spring 2012 Stress and Equilibrium Concept Questions #2 - CorrectionsDocument12 pages16.20 - Structural Mechanics Spring 2012 Stress and Equilibrium Concept Questions #2 - CorrectionsMehmet MehmetNo ratings yet
- Ideal GasnDocument37 pagesIdeal GasnabdiiNo ratings yet
- Mat112-F Activity 7 Constructivism Lesson ActivityDocument2 pagesMat112-F Activity 7 Constructivism Lesson ActivityMikaela MabazzaNo ratings yet
- Load CombinationsDocument10 pagesLoad Combinationsramesh_madkatte1082No ratings yet
- Solucione de Problemas de ElectrodinamicaDocument4 pagesSolucione de Problemas de ElectrodinamicaFelipe HlNo ratings yet