Professional Documents
Culture Documents
Principles of Gas Nitriding 2
Uploaded by
anhntran4850Original Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Principles of Gas Nitriding 2
Uploaded by
anhntran4850Copyright:
Available Formats
Principles of Gas Nitriding (Part 2)
by Daniel H. Herring
May 3, 2011
Editors note: This is the third of a four-part
presentation, including an online-exclusive article in April. In an effort
to establish a logical order, we will label figures consecutively as they
appear, including the online content. Consequently, the numbering of
some figures may appear to be missing, depending on your starting
point.
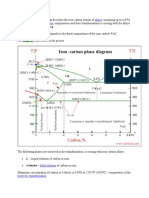
Nitriding is a case-hardening process in which nitrogen is introduced
into the surface of a ferrous alloy such as steel by holding the metal
at a temperature below that at which the crystal structure begins to
transform to austenite on heating (Ac
1
) as defined by the Iron-Carbon
Phase Diagram.
Fig. 14. Dissociation of ammonia and nitrogen
pickup in steel during gas nitriding[4]
Gas Nitriding Reactions
Gas nitriding is typically done using ammonia with
or without dilution of the atmosphere with dissociated ammonia or
nitrogen (or nitrogen/hydrogen) in the temperature range of 925-
1050F (500-565C). Ammonia (NH
3
) is allowed to flow over the
parts to be hardened.
Fig. 15. Nitriding surface and subsurface reactions[5]
Due to the temperature and the catalytic effect of the steel surface,
the ammonia dissociates into atomic nitrogen and hydrogen in
accordance with equation 1:
2NH
3
2N + 6 H (1)
This is immediately followed by atomic nitrogen combining to form
molecular nitrogen per equation 2:
2N + 6 H N
2
+ 3 H
2
(2)
During the period in which this nitrogen passes through the atomic
state, it is capable of being absorbed into the steel (Fig. 14).
So, the entire reaction equation 3, Figure 15 becomes:
2NH
3
N
2
+ 3 H
2
(3)
Gas Nitriding Activity
In accordance with the laws governing diffusion, the degree of
nitrogen penetration is governed by the temperature and the amount
of nitrogen that can penetrate and diffuse into and away from the
outer layer of the steel.
Fig. 16. Schematic representation of ammonia dissociation and
nitrogen absorption[5]
In gas nitriding, the nitrogen activity is controlled by the degree of
dissociation and the flow rate of the gas (Fig. 16). The nitrogen is
supplied by the dissociation of ammonia at the steel surface in
accordance with equation 4, a modified form of equation 1.
NH
3
[N] + 3/2 H (4)
By comparison with gas carburizing, the nitriding atmosphere is not
in equilibrium since the flow rate of ammonia is too high to allow
equilibrium to be achieved.
The amount of ammonia present in the outlet gas is a measure of the
degree of dissociation. The higher the flow rates of ammonia, the
higher the ammonia percentage in the exiting gas stream and the
lower the degree of dissociation. However, a greater percentage of
ammonia is present at the surface.
Equation 5 provides an explanation of the nitrogen activity in which
the activity constant (a
N
) is directly proportional to the degree of
ammonia dissociation and the flow rate.
a
N
a v (5)
where a
N
is the activity of atomic nitrogen
a is the degree of dissociation
v is the ammonia flow rate
Consequently, the nitrogen activity is a function of the number of
ammonia molecules dissociated at the steel surface per unit of time.
At constant pressure and temperature, the degree of dissociation is
reduced as the flow rate increases, but the product (a v) increases
and so does aN.
Thus, the nitrogen potential (KN) derived from equation 4 can be
expressed as:
K
N
= pNH
3
/ (pH
2
)
3/2
(6)
The amount of white layer can be controlled by minimizing the
nitriding potential. AMS 2759/10 (Automated Gaseous Nitriding
Controlled by Nitriding Potential) indicates nitriding potential values
(Table 5) for the various classes of white layer.
Nitrogen potential is also referred to as the
nitriding parameter. At a constant temperature,
the nitrogen activity, and consequently the nitrogen content, at the
surface of the nitrided surface layer are determined by the nitriding
potential. The various phases formed are expressed in the Lehrer
Diagram (Fig. 17).
Fig. 17. Lehrer relationship between nitriding potential and the phase
formed within the compound layer[4]
It is also important to guarantee that there is an adequate amount of
nitrogen available during the process to harden the parts to
specification. If there is not enough nitrogen available, the
consequence will be low case depths and hardness, with related
reductions in physical characteristics.
On the other hand, too much nitrogen at the part surface will result in
formation of a brittle and excessively thick white layer, resulting in
embrittlement of the nitrided case.
One of the keys to successful nitriding is controlling the percentage of
ammonia available per square area of (work) surface that will supply
atomic (nascent) nitrogen at the surface. It is important to realize
that nitriding is due only to the dissociation of ammonia at the part
surface, not due to the presence of molecular nitrogen (N
2
) or
dissociated ammonia (N
2
+ 3 H
2
).
The nitriding reaction (Eq. 1, 2) will ultimately go to completion, but
this is a very slow reaction. Empirical work has resulted in a rule of
thumb that says if the furnace atmosphere is changed four times
every hour, the amount of ammonia that is dissociated is 2510%.
An approximate relationship between ammonia flow rate and
percentage dissociation exists (Fig. 18). The general shape of the
curve will vary as a function of the furnace style, workload size and
surface area.
Fig. 18. Percentage dissociation as a function of furnace volume[6]
Hence, the best control method for the process is one that measures
and controls the percentage of ammonia. When we talk about a 30%
dissociation rate, we normally refer to a concentration of 70%
ammonia and 30% dissociated ammonia in the exhaust gas. In
reality, due to the volume change involved, only 82.3% is ammonia
while 17.7% is dissociated ammonia.
To nitride successfully, an adequate supply of atomic nitrogen must
be available at the part surface. Thus, in gas nitriding, it becomes
very important to circulate the ammonia in such a way as to
constantly resupply the active nitrogen on all areas to be hardened.
Gas Nitriding Cycles and Case-Depth Determination
Two types of nitriding processes are used: the
single-stage process and two-stage or Floe (pronounced flow)
process named after its inventor, Dr. Carl Floe.
Case-depth and case-hardness properties vary not only with the
duration and type of nitriding being performed but also with steel
composition, prior structure and core hardness. Case depths are
typically 0.008-0.025 inches (0.20-0.65 mm) and take 10-80 hours
to produce.
Single-Stage Nitriding Process
In the single-stage process, a temperature range of 925-975F (500-
525C) is typical. The dissociation rate of ammonia into nitrogen and
hydrogen ranges from 15-30%. The process produces a brittle,
nitrogen-rich layer known as the white layer (compound zone) at
the surface and is comprised of various iron nitrides (FeN, Fe
4
N,
Fe
16
N
2
).
Two-Stage Floe Process (U.S. Patent No. 2,437,249)
The two-stage process (Table 7 and 8) was developed to reduce the
amount of white layer formed by single-stage nitriding. The first
stage is, except for time, the same as that of the single-stage
process. In the second stage, however, the addition of a dilutant gas
(dissociated ammonia or nitrogen) increases the percent dissociation
to around 65-85%. The temperature is typically raised to 1025-
1075F (550-575C), and the result is the reduction of the depth of
the white layer, producing a deeper case of slightly lower hardness. If
the two-stage method is used, it is frequently possible to meet
dimensional tolerances without any final grinding operation.
Dissociated ammonia is generally required for high second-stage
dissociation (otherwise erratic control may result), and it is commonly
used as a dilutant (to change the percentage per square area that
NH
3
molecules are exposed to). In some cases, nitrogen is used.
However, white-layer control and porosity can be affected. Loading
arrangement and the use of a furnace circulating fan are very
important so that a high dissociation level may be achieved. The
nitrogen potential varies with the composition of the gas mixture that
is being sent into the furnace.
Crystal (Lattice) Structure
Ferrite, or alpha () iron, which is a body-centered
cubic (bcc) in crystal structure (Fig. 19), dissolves 0.001% nitrogen
at room temperature and 0.115% nitrogen at 1095F (590C).
Gamma prime (), or Fe4N, has a face-centered cubic (fcc) crystal
structure (Fig. 20) and dissolves 5.7-6.1% nitrogen. Fe
2
N and Fe
3
N
are called epsilon (), which has a hexagonal closed packed (hcp)
crystal structure and dissolves between 8.0% and 11.0% nitrogen.
Fig. 19. Body-centered cubic (bcc) crystal structure; Fig. 20. Face-
centered cubic (fcc) crystal structure
Control of the Nitriding Process
There are several methods of controlling the
nitriding process based on analysis of the
percentage of dissociation.
One method involves the use of an ammonia analyzer (Fig. 21),
which is tied into ammonia and dissociated ammonia (or nitrogen)
flowmeters (for use during the second stage of nitriding). Based on
the output from the ammonia analyzer, the process can be accurately
controlled.
Fig. 21. Ammonia control system (courtesy of Super Systems Inc.)
Another method used to measure the degree of dissociation is an
analysis of the amount of hydrogen in the exhaust gas (Fig. 22).
From equation 4 we see:
1 volume NH
3
1/2 volume
N
4
+ 3/2 volume H
4
(7)
Fig. 22. Anatomy of a hydrogen sensor[5]
For example, if the measured volume percentage of hydrogen is
30%, the volume percentage nitrogen is 10% (30/3), and the
remaining ammonia volume is 60% (100% 30% 10%). Given the
original volume of ammonia supplied () into the furnace chamber,
equation 8 allows us to calculate the degree of dissociation () in the
exhaust gas.
1 /100 = (1 /100)
[(1 /100 + 2(/100)] (8)
Instruments for in-situ measurement of the nitriding potential via the
hydrogen content (and other methods) are commercially available
and under development. These types of continuous-measurement
devices are especially important for the short cycles up to 20 hours.
Alternately, a manual method for the control of the nitriding
atmosphere involves the use of a dissociation pipette or burette (Fig.
23).
Fig. 23. Manual measurement of percentage dissociation[8]
Ammonia is completely soluble in water. When water is introduced
into the dissociation pipette, any ammonia present dissolves,
instantly forming ammonia hydroxide (NH
4
OH). Water continues to
enter until it occupies a volume equivalent to that previously occupied
by the ammonia. The remainder of the exhaust gas, being insoluble
in water, collects at the top of the pipette. The height of the water
level is read directly from the scale of graduations, and this reading
indicates the percentage of non-water-soluble hydrogen-nitrogen gas
in the sample.
This reading, although not completely accurate, is the degree of
dissociation. It should be noted that the dissociation of ammonia
involves a twofold increase in volume as shown in equation 3. IH
References available in Aprils online exclusive
For more information: Dan Herring is president of THE HERRING GROUP
Inc., P.O. Box 884 Elmhurst, IL 60126; tel: 630-834-3017; fax: 630-
834-3117; e-mail: heattreatdoctor@industrialheating.com; web:
www.heat-treat-doctor.com. Dans Heat Treat Doctor columns appear
monthly in Industrial Heating, and he is also a research associate
professor at the Illinois Institute of Technology/Thermal Processing
Technology Center.
Daniel H. Herring
You might also like
- 1.0 TitleDocument10 pages1.0 TitlezackziffiNo ratings yet
- Wedling ModelingDocument32 pagesWedling ModelingManish KumarNo ratings yet
- Timken Ball Bearings CatalogDocument126 pagesTimken Ball Bearings Catalogmohananc67No ratings yet
- Flame Spraying Gun Uni Spray JetDocument5 pagesFlame Spraying Gun Uni Spray JetCristobal MontalbaNo ratings yet
- Fundamentos de Metalurgia Física - VerhoevenDocument60 pagesFundamentos de Metalurgia Física - VerhoevenLeoncio Santos Tress100% (1)
- Non-Traditional Machining: Electro Chemical Machining (ECM)Document14 pagesNon-Traditional Machining: Electro Chemical Machining (ECM)NimoNo ratings yet
- Box Culverts - Humes BookletDocument17 pagesBox Culverts - Humes BookletScott Downs100% (3)
- Ecodial Advance Calculation 4.1Document33 pagesEcodial Advance Calculation 4.1Youwan LeeNo ratings yet
- Solution Manual For Designing and Managing The Supply Chain 3rd Edition by David Simchi LeviDocument56 pagesSolution Manual For Designing and Managing The Supply Chain 3rd Edition by David Simchi LeviOmnia MustafaNo ratings yet
- The Iron-Carbon Phase Diagram: Prof. H. K. Khaira Professor in MSME Deptt. MANIT, BhopalDocument38 pagesThe Iron-Carbon Phase Diagram: Prof. H. K. Khaira Professor in MSME Deptt. MANIT, BhopalYogesh KumbharNo ratings yet
- Bar Basics and TerminologyDocument24 pagesBar Basics and TerminologyEdwin MPNo ratings yet
- Physical Metallurgy M1 PDFDocument21 pagesPhysical Metallurgy M1 PDFAnca ElenaNo ratings yet
- ME-311 Machine Design - Lecture 6Document17 pagesME-311 Machine Design - Lecture 6Muhammad Zun Nooren BangashNo ratings yet
- Engineering Failure Analysis 11 (2004) 873-893 PDFDocument21 pagesEngineering Failure Analysis 11 (2004) 873-893 PDFKailas GophaneNo ratings yet
- 2 Cast Iron FatigueDocument47 pages2 Cast Iron FatigueBruno BrepohlNo ratings yet
- Lecture 10 Fracture MechanicsDocument21 pagesLecture 10 Fracture Mechanicsantoine demeire100% (3)
- Solidification, Phase Diagrams and Phase TransformationDocument35 pagesSolidification, Phase Diagrams and Phase TransformationkrishnasaiNo ratings yet
- Paper Manufacturing Technology by VoithDocument76 pagesPaper Manufacturing Technology by VoithMarcWorldNo ratings yet
- ME-218 Lecture 5Document17 pagesME-218 Lecture 5Hannan AyubNo ratings yet
- Testeo Transmisión 966HDocument9 pagesTesteo Transmisión 966HVictorNo ratings yet
- Lecture On Water Treatment Plant: M/S Jindal Power Limited (4X250 MW) Opjstpp, Tamnar, RaigarhDocument41 pagesLecture On Water Treatment Plant: M/S Jindal Power Limited (4X250 MW) Opjstpp, Tamnar, RaigarhPrudhvi RajNo ratings yet
- Flare System PDFDocument41 pagesFlare System PDFAzar Deen100% (1)
- Fatigue Test DesignDocument5 pagesFatigue Test Designamir_homNo ratings yet
- Jaw CrusherDocument8 pagesJaw Crushermeharii0% (1)
- SF6 Gas Insulated Switch Gear (GIS)Document12 pagesSF6 Gas Insulated Switch Gear (GIS)Rakesh Reddy100% (1)
- wk7 (3) - Fe-C SystemDocument12 pageswk7 (3) - Fe-C Systemsaeed khaledNo ratings yet
- Nadca 402 4aDocument44 pagesNadca 402 4awholenumberNo ratings yet
- ME-311 Machine Design - Lecture 3Document27 pagesME-311 Machine Design - Lecture 3Muhammad Zun Nooren BangashNo ratings yet
- Iron Carbon Phase DiagramDocument3 pagesIron Carbon Phase DiagramrabikmNo ratings yet
- Deformation Behavior of The Surface Defects of Low Carbon Steel in Wire Rod RollingDocument6 pagesDeformation Behavior of The Surface Defects of Low Carbon Steel in Wire Rod RollingAvinash KumarNo ratings yet
- Stress Concentration, Theoretical Stress Concentration Factor, Notch Sensitivity FactorDocument64 pagesStress Concentration, Theoretical Stress Concentration Factor, Notch Sensitivity FactorShiva TejNo ratings yet
- Chapter2 - AJMDocument13 pagesChapter2 - AJMravish kumarNo ratings yet
- Effect of Cooling Rate On Microstructure and Mechanical Properties of Gray Cast Iron - IsIDocument6 pagesEffect of Cooling Rate On Microstructure and Mechanical Properties of Gray Cast Iron - IsIgiokniessNo ratings yet
- Application Manual Chapter 6 - Feeding & GatingDocument148 pagesApplication Manual Chapter 6 - Feeding & GatingVishal MaliNo ratings yet
- Chap 6 TTT Diagram (New)Document26 pagesChap 6 TTT Diagram (New)eeit_nizamNo ratings yet
- Ass 1 Mech 6511 Mechanical Shaping of Metals and PlasticsDocument2 pagesAss 1 Mech 6511 Mechanical Shaping of Metals and PlasticsVarinder ThandiNo ratings yet
- Gear Heat TreatmentDocument13 pagesGear Heat TreatmentvishalNo ratings yet
- ME 292 - Metallic Materials SessionalDocument39 pagesME 292 - Metallic Materials SessionalMuhammedNayeemNo ratings yet
- Leidenfrost EffectDocument10 pagesLeidenfrost EffectSnowswimmerNo ratings yet
- An Experimental Study To Corelate Water Jet Impingement Erosion Resistance and Properties of MetallicDocument12 pagesAn Experimental Study To Corelate Water Jet Impingement Erosion Resistance and Properties of MetallicsaatehNo ratings yet
- The Current State of Worldwide Standards of Ductile IronDocument8 pagesThe Current State of Worldwide Standards of Ductile IronN.PalaniappanNo ratings yet
- Evolution of Quench Factor Analysis - A ReviewDocument22 pagesEvolution of Quench Factor Analysis - A Reviewluigi_mazzucco100% (1)
- Piercing of Low-Carbon SteelDocument3 pagesPiercing of Low-Carbon SteelRicardo PaceNo ratings yet
- CleanSteel9 2015 CAPURRO PDFDocument11 pagesCleanSteel9 2015 CAPURRO PDFSpark Fernando Calderon ContrerasNo ratings yet
- Gmaw PDFDocument8 pagesGmaw PDFsekhar_ntpcNo ratings yet
- Ni ResistDocument2 pagesNi ResistAslan AlpNo ratings yet
- A Comparative Study of The Forming-Limit Diagram Next Term Models For Sheet SteelsDocument8 pagesA Comparative Study of The Forming-Limit Diagram Next Term Models For Sheet SteelsRaghav KhajuriaNo ratings yet
- Diagram EllinghamDocument9 pagesDiagram EllinghamcindycinpengNo ratings yet
- Tutorial No 4 TorsionDocument7 pagesTutorial No 4 TorsionwaleedkhalillahmedNo ratings yet
- RMW3 20 Harata TDocument18 pagesRMW3 20 Harata TmaghfiraNo ratings yet
- Applications of WeldingDocument14 pagesApplications of WeldingFarah NazNo ratings yet
- Forging of Specific Metals And: AlloysDocument13 pagesForging of Specific Metals And: AlloysKasia MazurNo ratings yet
- Tugas 5 Weld Metal Solidification & MicrostructureDocument16 pagesTugas 5 Weld Metal Solidification & MicrostructureDeepakNo ratings yet
- Ductile Iron ReviewDocument36 pagesDuctile Iron ReviewDenis Yasmin AlineNo ratings yet
- 01 - Fundamentals of Metalworking-2017son01Document92 pages01 - Fundamentals of Metalworking-2017son01emreNo ratings yet
- 2c1 Seam WeldingDocument57 pages2c1 Seam WeldingBasavarajBusnurNo ratings yet
- All ProblemsDocument107 pagesAll ProblemsjoshiabhijeetNo ratings yet
- 10 - Fundamentals of Metal Forming (Chapter 14)Document37 pages10 - Fundamentals of Metal Forming (Chapter 14)Taher al suhamiNo ratings yet
- Effect of Microstructure and Alloy Contents On The Luders Line Formation in Al-Mg AlloysDocument6 pagesEffect of Microstructure and Alloy Contents On The Luders Line Formation in Al-Mg AlloysJinsoo KimNo ratings yet
- Determination of The Paris Curve For A TWIP Steel For Body-in-White ApplicationsDocument94 pagesDetermination of The Paris Curve For A TWIP Steel For Body-in-White ApplicationsMatteo de'Giovanetti100% (1)
- Soft Annealing Heat Treatment PDFDocument6 pagesSoft Annealing Heat Treatment PDFsivajirao70No ratings yet
- MATE1000 Lecture 12 Strengthening Mechanisms in MetalsDocument4 pagesMATE1000 Lecture 12 Strengthening Mechanisms in MetalsclearcastingNo ratings yet
- Metal Forming v1 1Document39 pagesMetal Forming v1 1Gosaye DesalegnNo ratings yet
- Internal Expanding Rim Clutches & Brakes: (A) Clutch (B) BrakeDocument20 pagesInternal Expanding Rim Clutches & Brakes: (A) Clutch (B) BrakeALINo ratings yet
- MartensiteDocument2 pagesMartensitemp87_ingNo ratings yet
- Deformation & Strengthening Mechanisms: Issues To Address..Document48 pagesDeformation & Strengthening Mechanisms: Issues To Address..Christopher MurilloNo ratings yet
- 07 Fracture 1-18 PDFDocument18 pages07 Fracture 1-18 PDFDepi WayatiNo ratings yet
- Production Gas Carburising: The Pergamon Materials Engineering Practice SeriesFrom EverandProduction Gas Carburising: The Pergamon Materials Engineering Practice SeriesNo ratings yet
- The Physical Metallurgy of Fracture: Fourth International Conference on Fracture, June 1977, University of Waterloo, CanadaFrom EverandThe Physical Metallurgy of Fracture: Fourth International Conference on Fracture, June 1977, University of Waterloo, CanadaD.M.R. TaplinNo ratings yet
- Nitrogen Gas Extinguisher System As A Countermeasure Against A Sodium Fire at MonjuDocument9 pagesNitrogen Gas Extinguisher System As A Countermeasure Against A Sodium Fire at MonjuJuhar MohammedNo ratings yet
- JWST DTA Stepper Motor Anomaly and Lessons Learned-7!23!15Document8 pagesJWST DTA Stepper Motor Anomaly and Lessons Learned-7!23!15anhntran4850No ratings yet
- Ni Hms 584775Document13 pagesNi Hms 584775anhntran4850No ratings yet
- JWST Brushless DC Motor Characteristics Analysis 2-5-2016Document13 pagesJWST Brushless DC Motor Characteristics Analysis 2-5-2016anhntran4850No ratings yet
- PageManager9 1Document1 pagePageManager9 1anhntran4850No ratings yet
- Effects of Bearing Cleaning Peter WardDocument16 pagesEffects of Bearing Cleaning Peter Wardanhntran4850No ratings yet
- Space Station Control Moment Gyroscope Lessons LearnedDocument15 pagesSpace Station Control Moment Gyroscope Lessons Learnedanhntran4850No ratings yet
- 3-1-2 Guo Bearing Stiffness PDFDocument26 pages3-1-2 Guo Bearing Stiffness PDFanhntran4850No ratings yet
- General Static Load Capacity in Slewing Bearings. Unified Theoretical Approach For Crossed Roller Bearings and Four Contact Point Angular Ball BearingsDocument7 pagesGeneral Static Load Capacity in Slewing Bearings. Unified Theoretical Approach For Crossed Roller Bearings and Four Contact Point Angular Ball Bearingsanhntran4850No ratings yet
- Stepper Motor KramerDocument46 pagesStepper Motor Krameranhntran4850No ratings yet
- Shock and Vibration Testing of An Amb Supported Energy Storage FlywheelDocument6 pagesShock and Vibration Testing of An Amb Supported Energy Storage Flywheelanhntran4850No ratings yet
- Ancra 1500 Lbs 425 Ksi 430ksi 52100 AllowableDocument10 pagesAncra 1500 Lbs 425 Ksi 430ksi 52100 Allowableanhntran4850No ratings yet
- Document Management and Filing SolutionDocument1 pageDocument Management and Filing Solutionanhntran4850No ratings yet
- BizCard XpressDocument1 pageBizCard Xpressanhntran4850No ratings yet
- Friction and Wear of Titanium Alloys Sliding Against Metal, Polymer, and Ceramic CounterfacesDocument9 pagesFriction and Wear of Titanium Alloys Sliding Against Metal, Polymer, and Ceramic Counterfacesanhntran4850No ratings yet
- Dry Friction Coefficient Versus Surface RoughnessDocument12 pagesDry Friction Coefficient Versus Surface Roughnessanhntran4850No ratings yet
- 1) Import The Stress Model Into TAS. Save The Model As A TAS ModelDocument4 pages1) Import The Stress Model Into TAS. Save The Model As A TAS Modelanhntran4850No ratings yet
- PageManager9 1Document1 pagePageManager9 1anhntran4850No ratings yet
- Coek - Info - Acorga Znx50a New Selective Reagent For The SolvenDocument16 pagesCoek - Info - Acorga Znx50a New Selective Reagent For The SolvenGeorgi SavovNo ratings yet
- Batch - Sheet 4.00Document2 pagesBatch - Sheet 4.00DEBABRATA SASMALNo ratings yet
- RB - Specifications Piping MaterialsDocument21 pagesRB - Specifications Piping MaterialsFalcon PeregrinusNo ratings yet
- Deogiri Electronics Cluster Technical PresentationDocument23 pagesDeogiri Electronics Cluster Technical Presentationvikas BNo ratings yet
- SSC 6mo Spec Sheet PDFDocument2 pagesSSC 6mo Spec Sheet PDFsiswoutNo ratings yet
- Advanced Process Design in High Volume Kneader Reactors Using Multiple Feed Ports To Avoid Crust Forming Foaming and Low Heat TransferDocument10 pagesAdvanced Process Design in High Volume Kneader Reactors Using Multiple Feed Ports To Avoid Crust Forming Foaming and Low Heat TransferRaja WajahatNo ratings yet
- Optitome 15: Automatic Oxyacetylene and Plasma-Arc Cutting EquipmentDocument8 pagesOptitome 15: Automatic Oxyacetylene and Plasma-Arc Cutting EquipmentFlamur HasaniNo ratings yet
- Astm A358 A358mDocument11 pagesAstm A358 A358mShashank SaxenaNo ratings yet
- Innermost - Main CatalogueDocument214 pagesInnermost - Main CatalogueLiteHouseNo ratings yet
- XRAY NT18 Engine Instruction & Safety ManualDocument26 pagesXRAY NT18 Engine Instruction & Safety ManualAsierReloopNo ratings yet
- Huawei FM1000A40 V200R003C00 IT Maintenance GuideDocument179 pagesHuawei FM1000A40 V200R003C00 IT Maintenance GuideJesusNo ratings yet
- Hot & Cold WorkingDocument18 pagesHot & Cold WorkingMadushan MadushaNo ratings yet
- As 1074-1989 Steel Tubes and Tubulars For Ordinary ServiceDocument7 pagesAs 1074-1989 Steel Tubes and Tubulars For Ordinary ServiceSAI Global - APAC50% (2)
- JISDocument30 pagesJISImam SalehNo ratings yet
- Barry Bcdi All Elastomer IsolatorsDocument10 pagesBarry Bcdi All Elastomer IsolatorsdouglascoombsNo ratings yet
- 2014 Hyundai Equus 90049Document479 pages2014 Hyundai Equus 90049Anonymous bH1GoYuNo ratings yet
- Cableado IndustrialDocument16 pagesCableado IndustrialzerohuntercodeNo ratings yet
- Chem Lab Report 9:26:18Document3 pagesChem Lab Report 9:26:18Andrew SmithNo ratings yet
- Functional Specification For Three Phase Peak Substation Distribution Transformers 300 12000 Kva Ps202010enDocument13 pagesFunctional Specification For Three Phase Peak Substation Distribution Transformers 300 12000 Kva Ps202010enFrancisco José Murias DominguezNo ratings yet
- FM5178R6 TBM Series EnglishDocument2 pagesFM5178R6 TBM Series EnglishalejgonzNo ratings yet
- Silicon Wafer Wetability On Metal Thin Film MorphologyDocument8 pagesSilicon Wafer Wetability On Metal Thin Film MorphologyLee chong looNo ratings yet