Professional Documents
Culture Documents
Distribución de Los Pares de Electrones Vsepr Ejemplos: Charge - 2
Uploaded by
MokhtarBensaid0 ratings0% found this document useful (0 votes)
16 views1 pageS2O3 has a charge of -2. It has a trigonal bipyramidal molecular geometry with sulfur at the center and five total bonding sites. Three oxygen atoms occupy the equatorial positions and two occupy the axial positions.
Original Description:
Original Title
2 Reactions form.docx
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentS2O3 has a charge of -2. It has a trigonal bipyramidal molecular geometry with sulfur at the center and five total bonding sites. Three oxygen atoms occupy the equatorial positions and two occupy the axial positions.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
16 views1 pageDistribución de Los Pares de Electrones Vsepr Ejemplos: Charge - 2
Uploaded by
MokhtarBensaidS2O3 has a charge of -2. It has a trigonal bipyramidal molecular geometry with sulfur at the center and five total bonding sites. Three oxygen atoms occupy the equatorial positions and two occupy the axial positions.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 1
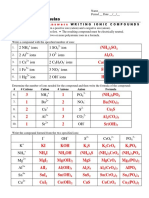
S2O3 Charge = -2
Distribución de los pares de VSEPR Ejemplos
electrones
Lineal AB2 BeCl2, CO2, CS2, COS, HCN, C2H2
Triangular Plana AB3 BF3, BCl3, SO3, H2CO, COCl2, C2H4,
C2H2F2, (CO3)2-
AB2E SnCl2, SO2, O3, NSF
Tetraédrica AB4 CH4, SiF4, (NH4)+, POF3, POCl3, SNF3, (SO4)2-, (S2O3)2-
AB3E NH3, PH3, AsH3, PF3, PCl3, PBr3, PI3, (H3O)+
AB2E2 H2O, H2S, Cl2O, OF2, (NH2)-
Bipirámide Trigonal AB5 PCl5, PF3Cl2, XeO3F2, PF3Cl2, SOF4, (IO5)3-
AB4E SF4, (IO2F2)-, XeO2F2
AB3E2 ClF3
AB2E3 (I3)-, XeF2
Octaédrica AB6 SF6, IOF5
AB5E BrF5, XeOF4
AB4E2 XeF4, (ICl4)-
You might also like
- Test Questions 2009Document69 pagesTest Questions 2009Dana CapbunNo ratings yet
- Solubility Table Worksheet PDFDocument2 pagesSolubility Table Worksheet PDFCed Hernandez100% (1)
- The Modern Spanish: Breyer and Zaitsev SystemsFrom EverandThe Modern Spanish: Breyer and Zaitsev SystemsRating: 5 out of 5 stars5/5 (1)
- Lewis Structures and Molecular GeometryDocument2 pagesLewis Structures and Molecular GeometryrsleoNo ratings yet
- Worksheet - Solubility Rules With AnswersDocument2 pagesWorksheet - Solubility Rules With AnswersEmmani HaginsNo ratings yet
- COLOUROf IONICCOMPOUNDSDocument2 pagesCOLOUROf IONICCOMPOUNDSkrutika goharkarNo ratings yet
- Phchem 1B - Quiz #1 - Chemical Nomenclature (Summer 2022)Document2 pagesPhchem 1B - Quiz #1 - Chemical Nomenclature (Summer 2022)Shopifyy ClothingNo ratings yet
- Cajepe, Cherry May F. Bses 1a ChemistryDocument4 pagesCajepe, Cherry May F. Bses 1a ChemistryNilda FranciscoNo ratings yet
- CH 6 Name For The Formula ShownDocument1 pageCH 6 Name For The Formula Showntownsenr94No ratings yet
- Formula Writing - CambridgeDocument5 pagesFormula Writing - CambridgeQusai Saify100% (3)
- Bond Angle Practice SheetDocument3 pagesBond Angle Practice Sheetgaurav100% (1)
- ChemicalBonding Assignment-2Document1 pageChemicalBonding Assignment-2Bitan DasNo ratings yet
- Problem Set No. 1Document3 pagesProblem Set No. 1Ej Ferrer100% (1)
- Volumetric Analysis - Class Assignment Part 01 PDFDocument2 pagesVolumetric Analysis - Class Assignment Part 01 PDFyug agarwalNo ratings yet
- Oxidation and Reduction-1 (13Document1 pageOxidation and Reduction-1 (13Aditya ChudasamaNo ratings yet
- Teri C No. Basic Geometry 0 Lone Pair 1 Lone Pair 2 Lone Pairs 3 Lone PairsDocument5 pagesTeri C No. Basic Geometry 0 Lone Pair 1 Lone Pair 2 Lone Pairs 3 Lone PairsShamsiNo ratings yet
- Syed ArsalanDocument1 pageSyed ArsalanShahzaib MughalNo ratings yet
- Ox. No & StateDocument2 pagesOx. No & StateajaxNo ratings yet
- Chemical BondingDocument4 pagesChemical BondingSARVESH PATILNo ratings yet
- Chemical Bonding - Sheet: 5 & 6 Level - 1: Page 1 of 4 CPP - Sankalp - Cb-5&6-Ph-IiDocument4 pagesChemical Bonding - Sheet: 5 & 6 Level - 1: Page 1 of 4 CPP - Sankalp - Cb-5&6-Ph-IigginrearrangeitproperlyNo ratings yet
- Chemical Formula & Names (Kamilia's Work)Document3 pagesChemical Formula & Names (Kamilia's Work)aina zahraaNo ratings yet
- TUpload 2Document1 pageTUpload 2Burikaw GamingNo ratings yet
- Class04 Chemistry G11 Homework Sep 25-29Document4 pagesClass04 Chemistry G11 Homework Sep 25-29Erin100% (1)
- Unit 7 Homework - Chemistry11Document10 pagesUnit 7 Homework - Chemistry11NameNo ratings yet
- Calventas Lab ReportDocument5 pagesCalventas Lab ReportGodwayneNo ratings yet
- Anion Schematic DiagramDocument1 pageAnion Schematic DiagramAlfie16No ratings yet
- 2022 2023 General Chemistry I Study Question Set 2Document1 page2022 2023 General Chemistry I Study Question Set 2Ömer Burak YükselNo ratings yet
- Formula WriterDocument1 pageFormula WriterswapnilNo ratings yet
- Eleazar - Quiz#3Document2 pagesEleazar - Quiz#3ゆかりNo ratings yet
- IonicBonding WritingFormulas WKST KEYDocument2 pagesIonicBonding WritingFormulas WKST KEYMaria Isabel DicoNo ratings yet
- 2 - Balancing Equations (Model Answer)Document1 page2 - Balancing Equations (Model Answer)ahmedsaherNo ratings yet
- JRS Tutorials: Chemistry IITDocument58 pagesJRS Tutorials: Chemistry IITtusharr11.mobNo ratings yet
- 7 - Chemical Formulas: AnswersDocument1 page7 - Chemical Formulas: AnswersAlyssa Mae MayonadoNo ratings yet
- Naming & Balancing Chemical Formula - Sheet1Document1 pageNaming & Balancing Chemical Formula - Sheet1arseniy kraschenkoNo ratings yet
- Unacademy - IOCXII MegaDPP 23withoutDocument2 pagesUnacademy - IOCXII MegaDPP 23withoutAaryan KeshanNo ratings yet
- Cations/anions CL CO NO S PO CN Na NH MG Al PBDocument3 pagesCations/anions CL CO NO S PO CN Na NH MG Al PBJohnmarco RomeroNo ratings yet
- Exp1 Prelab Day1Document2 pagesExp1 Prelab Day1Aaron OrtegaNo ratings yet
- WKS Mixed Formulas #1Document1 pageWKS Mixed Formulas #1laliberte68No ratings yet
- SO C H O PO NO Al: Writing Formulas & Naming CompoundsDocument2 pagesSO C H O PO NO Al: Writing Formulas & Naming Compoundsmaanoayumi.icctNo ratings yet
- Coordination Chemistry Sheet 4 IUPACDocument3 pagesCoordination Chemistry Sheet 4 IUPACAtharva MaheshwariNo ratings yet
- Mixed FormulasDocument3 pagesMixed FormulasasierNo ratings yet
- Lewis StructureDocument1 pageLewis StructureJay playNo ratings yet
- CW 4 Unit 5 - Chemical FormulaDocument1 pageCW 4 Unit 5 - Chemical Formulamohammad hasanNo ratings yet
- Chemical Compound List WikiDocument152 pagesChemical Compound List Wikismruti sangitaNo ratings yet
- SCH3U0 Nomenclature PracticeDocument7 pagesSCH3U0 Nomenclature PracticeArmann JohalNo ratings yet
- Assignment Colour Compound (Mega) 215Document2 pagesAssignment Colour Compound (Mega) 215Anant JainNo ratings yet
- Activity No. 3 Nomenclature of Inorganic Compounds Classify The Following As An Oxide, Acid, Base, or Salt. (1 PT Each)Document2 pagesActivity No. 3 Nomenclature of Inorganic Compounds Classify The Following As An Oxide, Acid, Base, or Salt. (1 PT Each)Alyssa Crizel CalotesNo ratings yet
- Fall 2021/STEM1-Chemistry/Worksheet 5/chapter 2.6-2.7/Dr. LingDocument3 pagesFall 2021/STEM1-Chemistry/Worksheet 5/chapter 2.6-2.7/Dr. LingMohamed alharthiNo ratings yet
- COLOUR OF ALL IOC COMPOUNDS @HeyitsyashXDDocument2 pagesCOLOUR OF ALL IOC COMPOUNDS @HeyitsyashXDzehraNo ratings yet
- Chemical FormulaDocument52 pagesChemical FormulaKalai VillaNo ratings yet
- Answer Key - Exam Review - Dec 2022 - ChemistryDocument14 pagesAnswer Key - Exam Review - Dec 2022 - Chemistrynicolas.randaxheNo ratings yet
- CHM 201 2019-2020 Note1Document38 pagesCHM 201 2019-2020 Note1Adams TemitopeNo ratings yet
- HKKX Fo"K Kred Iz'U: Part - I: Subjective QuestionsDocument10 pagesHKKX Fo"K Kred Iz'U: Part - I: Subjective QuestionswanderedNo ratings yet
- Chemical Formula Writing Worksheet PDFDocument4 pagesChemical Formula Writing Worksheet PDFkezia0% (1)
- Salt Analysis (Answer) (12th)Document16 pagesSalt Analysis (Answer) (12th)Raju SinghNo ratings yet
- Formula Zio ADocument9 pagesFormula Zio AJara EspumosaNo ratings yet
- All Exceptions in IOCDocument30 pagesAll Exceptions in IOCKalyan Reddt100% (2)
- Mineral 4Document90 pagesMineral 4Andres Gallardo LoayzaNo ratings yet
- Chemical Bonding WorksheetDocument2 pagesChemical Bonding WorksheetRong CaoNo ratings yet