Professional Documents
Culture Documents
What Is Thermodynamics?: Convertible Forms of Energy - Conservation of Energy
Uploaded by
Farouk Bassa0 ratings0% found this document useful (0 votes)
5 views17 pagesOriginal Title
introduction.docx
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
5 views17 pagesWhat Is Thermodynamics?: Convertible Forms of Energy - Conservation of Energy
Uploaded by
Farouk BassaCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 17
Introduction
What is Thermodynamics?
Deals with interrelationship between thermal energy
and mechanical energy – heat, work and the properties
of the system
Heat engine – operating in a cyclic manner produces
net work from a supply of heat
Laws of Thermo - natural hypothesis based on
observation
Basis of first law is heat and work is mutually
convertible forms of energy - conservation of energy
- introduces the concept of internal energy.
second law dictates limits on the conversion of heat
into work - measures performance – feasibility of a
particular process and specifies direction in which a
process will proceed - introduces entropy.
Laws of Thermodynamics in Lay Terminology
First Law: impossible to obtain something from
nothing, but may break even
Second Law: may break even but only at the lowest
possible temperature
Third Law: cannot reach the lowest possible temp
Concepts and Definitions
Pressure
Definition Pressure Force
Area
Units p N
m2
Derived unit, Pascal, Pa 1Pa 1N
m2
1 kPa = 103 N/m2; 1 MPa = 106 N/m2
1 bar = 105 N/m2
1 atm (standard atmosphere) = 101.325 kPa
Other: mm HG, torr (1 mmHG), m H2O, lbf/in.2 (psi)
1 Atm = 14.696 psi
Types of Pressure
- Absolute pressure, p (or pabs )
- Gage pressure, pgage
- Atmospheric pressure, patm (also termed ambient
pressure or barometric pressure)
- Vacuum pressure, pvac
absolute pressure (kPa); pressure measured relative to
a perfect vacuum - MUST be used in thermo relations.
gauge pressure (kPa,g). measured relative to
atmospheric pressure (≈100 kPa) - almost all pressure
gauges register zero when open to the atmosphere -
measure the difference between the pressure of fluid to
which connected and the surrounding air.
system pressure > atmospheric pressure - gage pressure
pgage = p – patm
atm pressure > system pressure - vacuum pressure
pvac = patm – p
Specific volume (v)
of a substance is the total volume (V) divided by total
mass (m) of that substance (volume per unit mass) -
units cubic meter per kilogram (𝑚3 ⁄𝑘𝑔).
𝑉 1
𝑣= =
𝑚 𝜌
Specific volume is preferred for thermo analysis when
working with gases - small density values
Heat, Work and the System
Energy
capacity of a system to perform work or produce heat
energy can be stored within systems in three
macroscopic forms: internal energy, kinetic energy, and
gravitational potential energy.
Energy also can be transferred to and from systems -
transferred to and from closed systems by two means
only: work and heat transfer. Work and heat transfer
are identified at the system boundary and are not
properties.
Heat
Energy transfer by heat to or from a system is due to
temp difference bet the system and its surroundings -
occurs in the direction of decreasing temp.
Heat cannot be contained in or possessed by a system
Thermodynamic Systems and Processes
Defining an appropriate system can greatly simplify a
thermodynamic analysis.
A thermo system is any 3-d region of space bounded by
one or more surfaces - bounding surfaces may be real
or imaginary, at rest or in motion, may change its size
or shape.
The region of physical space that lies outside the
selected boundaries of the system is called the
surroundings or the environment
Determining the boundary to solve a thermo problem
for a system depends on what information is known
and what question is asked about the system
Types of Thermodynamic Systems
isolated, closed, or open based on possible transfer of
mass and energy across the system boundaries.
isolated system – not influenced in any way by the
surroundings - no energy in the form of heat or work
may cross the boundary of the system. In addition, no
mass may cross the boundary of the system.
closed system - always contains the same matter (same
mass) - no transfer of mass with surroundings, may
have a transfer of energy (either heat or work)
open system - may have a transfer of both mass and
energy with its surroundings.
Work
Work is defined for mechanical systems as the action
of a force on an object through a distance - equals the
product of the force (F) times the displacement (d).
W = F•d
units N•m
Thermo definition of work extends notion of work
from mechanics to include other types of work.
boundary of closed system moves in direction of force
acting on it – surroundings do work on the system
boundary moves outward – work done by the system on
surroundings
Kinetic, potential, internal, and P-V energy are forms
of energy that are properties of a system.
Work is a form of energy, but it is energy in transit.
Work is not a property of a system. Work is a process
done by or on a system, but a system contains no work.
distinction bet forms of energy that are properties of a
system and forms of energy that are transferred to and
from a system is important to the understanding of
energy transfer systems.
Sign conventions for work and heat transfer:
all external inputs to a system are positive
Heat supplied to a system, Q, is positive
Work input to a system,W, is positive
State
condition of a system as described by its properties.
state of a system described by pressure, temperature,
specific volume, specific internal energy, specific
enthalpy, specific entropy
state specified by providing values of a subset of its
properties (any 2 for a pure fluid). All other properties
can be determined in terms of these few.
Thermodynamic Processes
A transformation from one state to another.
any property of a system changes - state changes -
system has undergone a process
for a pure fluid
other diagrams – temp vs entropy, enthalpy vs
entropy, press vs enthalpy
Reversible Process
A series of infinitesimal changes of state with
equilibrium established after each change - can be
represented by a line on a state diagram
Irreversible (Non-Equilibrium) Process
A finite change of state without equilibrium except at
end states.
It should not be represented by a line
Reversible v. Irreversible
reversible thermo process could be made to occur in
precisely reverse order, so that the system and all
associated systems would be returned from their final
condition to the conditions that existed before the
process started
could return all energy that was transformed or
redistributed during the process from its final to its
original form, amount and location
ALL REAL PROCESSES ARE IRREVERSIBLE TO
SOME DEGREE
for analysis purposes use reversible to make the
analysis simpler, and to determine maximum
theoretical efficiencies - reversible process starting
point on which to base eng study and calculation.
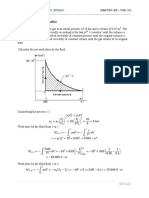
Reversible Work
under ideal conditions – ideal fluid, no friction
piston moves to the left
work done on the fluid by the surroundings
Work done, dW pA dl p dV mp dv
for reversible process state points joined to form line on
p-v diagram
W m 12 p dv m shaded area on figure
when reversible expansion takes place
W m 12 p dv m shaded area on figure
process from right to left on p-v diagram (contraction)
– work done on fluid - work input to the fluid – W is
+ve
process from left to right on p-v diagram (expansion) –
work done by fluid - work output to the fluid – W is –
ve
Cyclic Process
When a system in a given initial state goes through a
no. of different changes in state (going through various
processes) and finally returns to its initial values, the
system has undergone a cyclic process or cycle – at
conclusion of a cycle, all properties have the same
value they had at the beginning.
a reversible cycle plotted on a p-v diagram forms a
closed figure the area of which represents the net work
of the cycle
cycle 1 2 3 4 1 – net work input is shaded area
cycle 1 4 3 2 1 (reverse direction) – shaded area is net
work output
Rule: enclosed area
of a reversible cycle
represents net work
input (net work done
on the system) when
the cycle is anti-
clockwise.
You might also like
- Introduction To ThermodynamicsDocument16 pagesIntroduction To ThermodynamicsFarouk BassaNo ratings yet
- ChE 122 Lecture Notes 02 II. Basic Concepts and The First Law (2.1-2.6)Document4 pagesChE 122 Lecture Notes 02 II. Basic Concepts and The First Law (2.1-2.6)MarkVergelBorjaNo ratings yet
- RefDocument75 pagesRefagrawalvishal990% (1)
- Intro ThermodynamicsDocument55 pagesIntro Thermodynamicssunny1312No ratings yet
- Basic Electro MECHANICAL Engineering NotesDocument9 pagesBasic Electro MECHANICAL Engineering NotesMohsin Gondal100% (1)
- Thermodynamic Equilibrium and System PropertiesDocument4 pagesThermodynamic Equilibrium and System PropertiesRashid MinhasNo ratings yet
- Thermodynamics Basics and First LawDocument22 pagesThermodynamics Basics and First LawDamo Daran GNo ratings yet
- Thermofluids SummaryDocument6 pagesThermofluids SummaryneocentricgeniusNo ratings yet
- Introduction to Chemical Engineering Thermodynamics PrinciplesDocument18 pagesIntroduction to Chemical Engineering Thermodynamics PrincipleshusseinhshNo ratings yet
- TH - 4 Thermal Engineering-I: Chapter - 1 Thermodynamic Concept & TerminologyDocument20 pagesTH - 4 Thermal Engineering-I: Chapter - 1 Thermodynamic Concept & Terminologysaritprava sahooNo ratings yet
- Chapter 2: The First Law of Thermodynamics (Concepts)Document22 pagesChapter 2: The First Law of Thermodynamics (Concepts)arunyogNo ratings yet
- ThermodynamicsDocument17 pagesThermodynamicsMaster DeathNo ratings yet
- Unit 1Document31 pagesUnit 1araz_1985No ratings yet
- ME 6301 Engineering Thermodynamics NotesDocument39 pagesME 6301 Engineering Thermodynamics NotesamdevaNo ratings yet
- CHEMICAL THERMODYNAMICS-III (1) 1st SlideDocument41 pagesCHEMICAL THERMODYNAMICS-III (1) 1st SlideMuhammadObaidullahNo ratings yet
- Me 161: Introduction To Mechanicalengineering: Asif KabirDocument28 pagesMe 161: Introduction To Mechanicalengineering: Asif KabirMohammad Asif KabirNo ratings yet
- BasicsDocument65 pagesBasicsBas RamuNo ratings yet
- Eng Komolafe Thermodynamics Lecture Note. (Module 1-4) DocxDocument35 pagesEng Komolafe Thermodynamics Lecture Note. (Module 1-4) DocxOyedotun Tunde100% (1)
- BME - Module 1Document103 pagesBME - Module 1Pranav JayasuryaNo ratings yet
- ThermodynamicsDocument62 pagesThermodynamicsHarini MeiyappanNo ratings yet
- Thermodynamics: Basic DefinitionsDocument8 pagesThermodynamics: Basic DefinitionsAnu RadhaNo ratings yet
- Q & A - Basic ThermoDocument32 pagesQ & A - Basic ThermoManoranjan Kumar SinghNo ratings yet
- ThermodynamicDocument20 pagesThermodynamicDashanand RavanNo ratings yet
- Thermodynamics B Tech NotesDocument38 pagesThermodynamics B Tech NotesRajdeep ShawNo ratings yet
- 1st Law of ThermodynamicsDocument20 pages1st Law of ThermodynamicsellayuslianaNo ratings yet
- PIKEMDocument18 pagesPIKEMFlorenceNo ratings yet
- Basic Thermodynamics TheoryDocument13 pagesBasic Thermodynamics TheoryAmaresh Movies ASNo ratings yet
- CH I Concepts in ThermoDocument14 pagesCH I Concepts in ThermoIsaac S Whuling IIINo ratings yet
- Thermodynamics Lecture 1Document38 pagesThermodynamics Lecture 1MarkJude MorlaNo ratings yet
- ThermodynamicsDocument18 pagesThermodynamicseka123No ratings yet
- Unit 1Document39 pagesUnit 1ashwinharry69No ratings yet
- Basic Thermodynamics: Duthil@Document20 pagesBasic Thermodynamics: Duthil@gauravNo ratings yet
- BE 8256 - Basic Mechanical Engineering U-IDocument17 pagesBE 8256 - Basic Mechanical Engineering U-IarulrakkNo ratings yet
- Thermo LecturesDocument9 pagesThermo LecturesAhmad Ch100% (1)
- DEFINING THERMODYNAMICSDocument8 pagesDEFINING THERMODYNAMICSLis MiaNo ratings yet
- 22-23 JNV ThermodynamicsDocument76 pages22-23 JNV Thermodynamicsreadingchallenge jnvsklmNo ratings yet
- System and Surroundings: - SystemDocument19 pagesSystem and Surroundings: - SystemVighnesh ManojNo ratings yet
- Asic Mechanical Engineering: Unit-1 Part-1 ThermodynamicsDocument99 pagesAsic Mechanical Engineering: Unit-1 Part-1 ThermodynamicsSanjiv ParabNo ratings yet
- Mod1A Basics of ME ThermoDocument98 pagesMod1A Basics of ME ThermoGauthamanNo ratings yet
- Module 1Document82 pagesModule 1ALL TIME STUDYNo ratings yet
- Chapter 1 Jan2013Document30 pagesChapter 1 Jan2013enteryourname5No ratings yet
- Advanced Thermodynamics and Heat Engines: M SC in Mechanical System Design & Engineering, I/IDocument20 pagesAdvanced Thermodynamics and Heat Engines: M SC in Mechanical System Design & Engineering, I/ISandip GhimireNo ratings yet
- Module 1 NotesDocument33 pagesModule 1 NoteskrbautoNo ratings yet
- ME 6301 Engineering Thermodynamics Unit 1 Concepts and First LawDocument20 pagesME 6301 Engineering Thermodynamics Unit 1 Concepts and First LawDeepak MisraNo ratings yet
- Thermodynamics Theory EDocument45 pagesThermodynamics Theory Ethinkiit100% (1)
- Thermodynamics 02Document31 pagesThermodynamics 02Sachin BorseNo ratings yet
- The First Law and Other Basic Concepts PDFDocument64 pagesThe First Law and Other Basic Concepts PDFeyezakeyeNo ratings yet
- Surrounding. Anything Outside The System Which Affects The Behavior of The SystemDocument8 pagesSurrounding. Anything Outside The System Which Affects The Behavior of The SystemGopinath VNo ratings yet
- CERN 2014 005 p1Document20 pagesCERN 2014 005 p1Daejoong KimNo ratings yet
- Metallurgical Thermodynamics Notes 1Document16 pagesMetallurgical Thermodynamics Notes 1Nigel FaranandoNo ratings yet
- Thermodynamics: PressureDocument6 pagesThermodynamics: Pressurevinothenergy100% (1)
- Thermal EngineeringDocument10 pagesThermal EngineeringGanesh Datt SharmaNo ratings yet
- 1 Lecture (A Thermodynamic Review)Document12 pages1 Lecture (A Thermodynamic Review)Muhammad Ahmad Khan LodhiNo ratings yet
- Thermodynamics 2Document285 pagesThermodynamics 2John Lloyd A. VincoyNo ratings yet
- Defence Engineering College: Applied Thermodynamics MV2012Document38 pagesDefence Engineering College: Applied Thermodynamics MV2012Getachew TikueNo ratings yet
- Lecture Five Energy BalancesDocument51 pagesLecture Five Energy BalancesHebron DawitNo ratings yet
- ME291 Introduction to ThermodynamicsDocument11 pagesME291 Introduction to ThermodynamicsDaniel Deng KuolNo ratings yet
- Work and HeatDocument11 pagesWork and HeatRam AleNo ratings yet
- “Foundations to Flight: Mastering Physics from Curiosity to Confidence: Cipher 4”: “Foundations to Flight: Mastering Physics from Curiosity to Confidence, #4From Everand“Foundations to Flight: Mastering Physics from Curiosity to Confidence: Cipher 4”: “Foundations to Flight: Mastering Physics from Curiosity to Confidence, #4No ratings yet
- Distillation ControlDocument12 pagesDistillation ControlFarouk BassaNo ratings yet
- Steam Tables - SuperheatedDocument6 pagesSteam Tables - SuperheatedFarouk BassaNo ratings yet
- The Working Fluid in ThermodynamicsDocument13 pagesThe Working Fluid in ThermodynamicsFarouk BassaNo ratings yet
- The Diwan of Shaykh Muhammad Ibn Al-Habib Al-Amghari Al-Idrisi Al-HasaniDocument534 pagesThe Diwan of Shaykh Muhammad Ibn Al-Habib Al-Amghari Al-Idrisi Al-HasaniFarouk BassaNo ratings yet
- Applied Thermodynamics Exam 2018 Wirh SolutionsDocument9 pagesApplied Thermodynamics Exam 2018 Wirh SolutionsFarouk BassaNo ratings yet
- Question 1 (15 Marks)Document5 pagesQuestion 1 (15 Marks)Farouk BassaNo ratings yet
- Properties and Xy Diagram From ChemcadDocument4 pagesProperties and Xy Diagram From ChemcadFarouk BassaNo ratings yet
- Properties and Xy Diagram From ChemcadDocument4 pagesProperties and Xy Diagram From ChemcadFarouk BassaNo ratings yet
- Saturated Water Temperature TableDocument4 pagesSaturated Water Temperature TableFarouk BassaNo ratings yet
- Properties and Xy Diagram From ChemcadDocument5 pagesProperties and Xy Diagram From ChemcadFarouk BassaNo ratings yet
- Reversible and Irreversible ProcesesDocument12 pagesReversible and Irreversible ProcesesFarouk BassaNo ratings yet
- Introduction To Applid ThermodynamicsDocument17 pagesIntroduction To Applid ThermodynamicsFarouk BassaNo ratings yet
- Islamic Education Instead of AssimilationDocument18 pagesIslamic Education Instead of AssimilationFarouk BassaNo ratings yet
- Refrigeration and Heat PumpsDocument8 pagesRefrigeration and Heat PumpsFarouk BassaNo ratings yet
- First Law of ThermodynamicsDocument7 pagesFirst Law of ThermodynamicsFarouk BassaNo ratings yet
- Natural State. (Page 2)Document7 pagesNatural State. (Page 2)andrew_stewart_37No ratings yet
- Chem Eng PractsDocument10 pagesChem Eng PractsFarouk BassaNo ratings yet
- The Working Fluid in ThermodynamicsDocument13 pagesThe Working Fluid in ThermodynamicsFarouk BassaNo ratings yet
- Mu'awiya As A Model of Islamic Governance by Aisha Bewley PDFDocument15 pagesMu'awiya As A Model of Islamic Governance by Aisha Bewley PDFFarouk BassaNo ratings yet
- Early Governance in The Muslim UmmaDocument12 pagesEarly Governance in The Muslim UmmaFarouk BassaNo ratings yet
- Why HomeopathyDocument4 pagesWhy HomeopathyFarouk BassaNo ratings yet
- Unit Operations and FlowchartingDocument16 pagesUnit Operations and FlowchartingFarouk BassaNo ratings yet
- Natural State. (Page 2)Document7 pagesNatural State. (Page 2)andrew_stewart_37No ratings yet
- Liquefaction SlidesDocument37 pagesLiquefaction SlidesFarouk BassaNo ratings yet
- Tut - Control and Mathematical Modelling SolutionsDocument5 pagesTut - Control and Mathematical Modelling SolutionsFarouk BassaNo ratings yet
- Typical Control LoopsDocument11 pagesTypical Control LoopsFarouk BassaNo ratings yet
- Mu'awiya As A Model of Islamic Governance by Aisha Bewley PDFDocument15 pagesMu'awiya As A Model of Islamic Governance by Aisha Bewley PDFFarouk BassaNo ratings yet
- Tuts - Hazop AnswersDocument2 pagesTuts - Hazop AnswersFarouk BassaNo ratings yet
- Soln ProcedureDocument1 pageSoln ProcedureFarouk BassaNo ratings yet
- 4.1 The Plausibility of µ as a Value for a Normal Population Mean μDocument23 pages4.1 The Plausibility of µ as a Value for a Normal Population Mean μTolesa F BegnaNo ratings yet
- SYNOPSIS On Retail MarketingDocument23 pagesSYNOPSIS On Retail MarketingHariharasudan HariNo ratings yet
- Sist Elec. D6KDocument2 pagesSist Elec. D6KJose Luis Aguero PomachaguaNo ratings yet
- Project Time Management and Budget PlanningDocument68 pagesProject Time Management and Budget PlanningLindelani ndalaNo ratings yet
- Comparison of Rigid Pavement Thickness Design SystemsDocument168 pagesComparison of Rigid Pavement Thickness Design Systemskamalnath100% (1)
- Geological Exploration Flowchart and SumDocument1 pageGeological Exploration Flowchart and SumKareemAmenNo ratings yet
- Boiler Tube FailuresDocument15 pagesBoiler Tube FailuresBIRANCHINo ratings yet
- Linked PDFDocument337 pagesLinked PDFDmytro PichkurNo ratings yet
- Presentation Kaizen BaDocument87 pagesPresentation Kaizen BaAbela DrrsNo ratings yet
- Manual - de - Bolso - Fanuc 16i 18i 21i MODEL BDocument988 pagesManual - de - Bolso - Fanuc 16i 18i 21i MODEL BJeffsouza2016100% (4)
- 03 Preliminary PagesDocument12 pages03 Preliminary PagesBillie Jan Louie JardinNo ratings yet
- Surface Safety Products CatalogDocument37 pagesSurface Safety Products CatalogZaid AlahmedNo ratings yet
- Conveyor Control System ProjectDocument15 pagesConveyor Control System ProjectzhackhieNo ratings yet
- Linux Unit 4Document34 pagesLinux Unit 4Adeefa AnsariNo ratings yet
- Modern CV Template StyleDocument2 pagesModern CV Template StyleRedi Joel Córdova ArbietoNo ratings yet
- How To Deal With Multiple SAP Logons - Simple Excel VBADocument14 pagesHow To Deal With Multiple SAP Logons - Simple Excel VBAangel saezNo ratings yet
- Week - 5 (Deep Learning) Q. 1) Explain The Architecture of Feed Forward Neural Network or Multilayer Perceptron. (12 Marks)Document7 pagesWeek - 5 (Deep Learning) Q. 1) Explain The Architecture of Feed Forward Neural Network or Multilayer Perceptron. (12 Marks)Mrunal BhilareNo ratings yet
- Syllabus: For Probability and StatisticsDocument2 pagesSyllabus: For Probability and Statisticsshubham raj laxmiNo ratings yet
- Quick Start GuideDocument3 pagesQuick Start Guideswornavidhya.mahadevanNo ratings yet
- GSS5 Rising Main Condition Assessment and Risk Management ManualDocument2 pagesGSS5 Rising Main Condition Assessment and Risk Management ManualNickNo ratings yet
- DFX9000 Repair Manual PDFDocument223 pagesDFX9000 Repair Manual PDFRams ANo ratings yet
- Enumerators (x50) - Job Search MalawiDocument4 pagesEnumerators (x50) - Job Search MalawicliftonkacheremNo ratings yet
- G+1 Residential Building Bill of Quantity YITAYALDocument31 pagesG+1 Residential Building Bill of Quantity YITAYALYemane TsadikNo ratings yet
- Module 2 - Individual BehaviourDocument187 pagesModule 2 - Individual BehaviourBiswajeet DashNo ratings yet
- Croft LeafletDocument2 pagesCroft Leafletece142No ratings yet
- JAA ATPL BOOK 02 - Oxford Aviation - Jeppesen - Airframes and SystemsDocument421 pagesJAA ATPL BOOK 02 - Oxford Aviation - Jeppesen - Airframes and SystemsRicardo BorbaNo ratings yet
- Modeling For Simulation and Control of Acid-Base Neutralization SystemsDocument12 pagesModeling For Simulation and Control of Acid-Base Neutralization SystemscacacocoNo ratings yet
- Physical Preparation of The Modern Elite Football PlayerDocument8 pagesPhysical Preparation of The Modern Elite Football PlayerKamil SochaNo ratings yet
- Activity 3 GlobalizationDocument3 pagesActivity 3 GlobalizationLuis VillalobosNo ratings yet
- Survey of Old Mongolian Monasteries and Temples in UlaanbaatarDocument183 pagesSurvey of Old Mongolian Monasteries and Temples in UlaanbaatarUB OtgonNo ratings yet