Professional Documents
Culture Documents
Ai. (1971) - The Cone Pigment Has Been Only Bleached
Uploaded by
AkicaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ai. (1971) - The Cone Pigment Has Been Only Bleached
Uploaded by
AkicaCopyright:
Available Formats
VISUAL ADAPTATION AND RETINAL GAIN CONTROLS 287
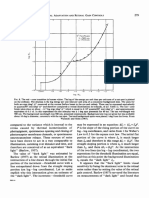
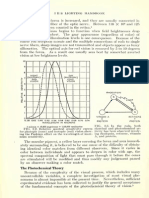
size (Biersdorf et al., 1965). This experiment for either rods or cones over much of the visual
indicates that the gain of the retina is affected at range of backgrounds (Rushton, 1962). This can be
the same light levels as the visual threshold for large seen in Figs. 15 and 16. Figure 15 is from Alpern
targets. A further question one might raise is, where and Pugh (1974) and shows the fraction of human
in the retina is the site of this gain change? Much rhodopsin, the rod pigment, bleached as a function
of the physiological work we present below was of a steady background. Even up to rod saturation
motivated by this question. Prior to the (5" 108 quanta(507 nm) (deg 2 s)-~) the rod pigment
physiological investigation, this question of the site has been bleached away less than 2°70, but the visual
in the retina of gain control was investigated threshold would have risen by a factor of more than
psychophysically and this work led to the two 107, a discrepancy in prediction. Figure 16 shows
concepts of gain control by pigment depletion, a similar result for human cones, from Alpern et
which has been shown to be only a minor factor ai. (1971). The cone pigment has been only bleached
in the control of retinal gain, and the adaptation by half at about 104s trolands which is near the top
pool, which still motivates research. of the photopic range. So we can rule out pigment
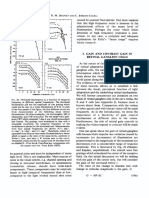
2.1.6.3. Pigment depletion. Vertebrate visual depletion as playing a major role in human light
pigments are bleached by light into non-receptive adaptation, though it has some effect on photopic
intermediates which must be regenerated to a (cone-driven) sensitivity (Boynton and Whitten,
receptive state enzymatically. It was thought for 1970; Valeton and van Norren, 1983). It is
some time that visual pigment depletion, resulting interesting that photopigment depletion began to be
from bleaching by the background light, was the doubted as an explanation of light adaptation quite
main cause of light adaptation (Hecht, 1924). This a long time ago, from experiments on increment
would put the site of adaptation right at the initial threshold and photopigment concentration in the
transduction stage in vision. However, it is now frog retina conducted by Granit et al. (1939). In
known that the pigment regenerates so quickly that those experiments ERG thresholds were increased
there is a rather small amount of pigment bleached by log units by backgrounds which had no
1.0
0.8 • :,\! '\ \ ; .
r, .-.\.
o
"~
o 0.6 .~ •.°
L-
575 n m
~ 0.4
"!\.
~°
0.2
500 n m
10 sec W h i t e
0 , I I I I I I I
12 13 14 15
Log q u a n t a sec -I c m -~ o f r e t i n a at t h e c o r n e a
FIG. 15. Pigment depletion in rods caused by steady illumination. The amount of the rhodopsin in a 5 deg field located
18 deg peripheral to the fovea in the temporal retina was measured with a retinal densitometer (described in Alpern et al.,
1971 and in Alpern and Pugh, 1974)• Plotted vertically is the fractional amount of rhodopsin left in the steady state at
the background illumination plotted on the horizontal scale. The three curves are for steady lights of 500 nm, 575 nm,
and white light• The 500 and 575 nm lights were obtained with the white source and interference filters• The results to
concentrate on are the 500 nm results, since 500 nm is near the peak wavelength of absorption of rhodopsin and so will
give the greatest pigment depletion at the lowest retinal illumination. The half bleaching point for the 500 nm curve is at
about 13.2 log quanta(cm 2 s)-' which is equivalent to about 10.2 log quanta(deg2 s)-1, the more familiar unit of retinal
illumination [also equivalent to about 4.3 log (scotopic) td]. These values of quanta are referred to the cornea and do not
take into account losses in the eye. From Alpern and Pugh 0974).
PRR3-H*
You might also like
- 0"4 I ' 0"2 4 Log I 2 Photopic TrofandsDocument1 page0"4 I ' 0"2 4 Log I 2 Photopic TrofandsAkicaNo ratings yet
- 2.1.1.1. Aguilar and Stiles' Experiment.: Visual Adaptation and Retinal G A I N Controls 2 7 7Document1 page2.1.1.1. Aguilar and Stiles' Experiment.: Visual Adaptation and Retinal G A I N Controls 2 7 7AkicaNo ratings yet
- Visual adaptation and retinal gain controlsDocument1 pageVisual adaptation and retinal gain controlsAkicaNo ratings yet
- Retina - 43Document1 pageRetina - 43AkicaNo ratings yet
- Mreznica - 59Document1 pageMreznica - 59AkicaNo ratings yet
- Retina - 24Document1 pageRetina - 24AkicaNo ratings yet
- I Ooo" ': IC) A ", I (D)Document1 pageI Ooo" ': IC) A ", I (D)AkicaNo ratings yet
- The Vibrational Spectra of Phenol and phenol-ODDocument11 pagesThe Vibrational Spectra of Phenol and phenol-ODMaulida Azizza ShizenNo ratings yet
- University of California Los Angeles .. - ,,-. - .: Approved For Release Cia-Rdp96-007B7R0004000Booic RDDocument6 pagesUniversity of California Los Angeles .. - ,,-. - .: Approved For Release Cia-Rdp96-007B7R0004000Booic RDChadNo ratings yet
- R - M - Shapley and C - Enroth-CugellDocument1 pageR - M - Shapley and C - Enroth-CugellAkicaNo ratings yet
- Tpeak: I - Dark Adapted - 4"4 BG - 3.2 BG - 2.1 BG I I IDocument1 pageTpeak: I - Dark Adapted - 4"4 BG - 3.2 BG - 2.1 BG I I IAkicaNo ratings yet
- Pbse BandstrucureDocument12 pagesPbse BandstrucurevinaychimalgiNo ratings yet
- Retina - 40Document1 pageRetina - 40AkicaNo ratings yet
- Abraham1988 PDFDocument4 pagesAbraham1988 PDFSRS 2016No ratings yet
- ApplOpt 38 1657 1999 MatrixInversionRetrievalDocument6 pagesApplOpt 38 1657 1999 MatrixInversionRetrievalZhao WenwenNo ratings yet
- Diffuse Reflectance Spectra and Optical Properties of Some Iron and Titanium Oxides and OxyhydroxidesDocument8 pagesDiffuse Reflectance Spectra and Optical Properties of Some Iron and Titanium Oxides and Oxyhydroxidesicpsdt.cuetNo ratings yet
- Ch17 Ch18 Review QuestionsDocument38 pagesCh17 Ch18 Review QuestionsLuis Rodriguez0% (1)
- Breaking Abbe's Diffraction Resolution Limit in Fluorescence MicrosDocument9 pagesBreaking Abbe's Diffraction Resolution Limit in Fluorescence Microspál locNo ratings yet
- OF of Stellar Spectra: Application DicyaninDocument25 pagesOF of Stellar Spectra: Application DicyaninLevicano100% (1)
- Rioja 1977Document5 pagesRioja 1977hcmadhuNo ratings yet
- Mreznica - 61Document1 pageMreznica - 61AkicaNo ratings yet
- The Human Visual System and Perception: / (A,) Pea,) L (A,)Document24 pagesThe Human Visual System and Perception: / (A,) Pea,) L (A,)kaiser yasserNo ratings yet
- Longtransient Effects in Lasers With Inserted Liquid SamplesDocument7 pagesLongtransient Effects in Lasers With Inserted Liquid SamplesDarwin GuangaNo ratings yet
- Research Department Tannoy Velocity Microphone: Report No. M.007 Serial NoDocument5 pagesResearch Department Tannoy Velocity Microphone: Report No. M.007 Serial NoalpNo ratings yet
- Retina 11Document1 pageRetina 11AkicaNo ratings yet
- Retina - 35Document1 pageRetina - 35AkicaNo ratings yet
- 1.reristivity Logging ToolDocument10 pages1.reristivity Logging ToolFatimah AzzahraNo ratings yet
- Fluctuations of Starlight and SkylightDocument26 pagesFluctuations of Starlight and Skylightskr2010No ratings yet
- Telecont: Single-Photon Counter ForDocument7 pagesTelecont: Single-Photon Counter ForDaliaBarrancoNo ratings yet
- Retina - 18Document1 pageRetina - 18AkicaNo ratings yet
- Maekawa1968 PDFDocument17 pagesMaekawa1968 PDFMarely Teresa Ruiz AntolinezNo ratings yet
- BF00576726Document2 pagesBF00576726AZIL KenzaNo ratings yet
- Confort Ingles 23Document1 pageConfort Ingles 23jade485No ratings yet
- 03 14 PerronDocument12 pages03 14 Perronlucille.bonnierNo ratings yet
- Base SchiffDocument10 pagesBase SchiffDánica Nicoll Rojas MolinaNo ratings yet
- Near-IR Spectroscopy Determines Epoxy Resin CureDocument11 pagesNear-IR Spectroscopy Determines Epoxy Resin CureRick MortyNo ratings yet
- Visual Adaptation and Retinal Gain ControlsDocument1 pageVisual Adaptation and Retinal Gain ControlsAkicaNo ratings yet
- Facts and Artifacts in Near-Field Optical Microscopy: Related ArticlesDocument8 pagesFacts and Artifacts in Near-Field Optical Microscopy: Related ArticlesClaudio BiaginiNo ratings yet
- PhysRevB.66.195415Document9 pagesPhysRevB.66.195415CH YNo ratings yet
- Prinsip HuygensDocument36 pagesPrinsip Huygensqc12345No ratings yet
- Colored InversionDocument5 pagesColored Inversiondanial bramantaNo ratings yet
- O / e (AT o'A'T) : Log SDocument1 pageO / e (AT o'A'T) : Log SAkicaNo ratings yet
- C. Enroth-Cugell: Et Al.Document1 pageC. Enroth-Cugell: Et Al.AkicaNo ratings yet
- Types of Fluorescence Transitions in Atomic Fluorescence SpectrometryDocument3 pagesTypes of Fluorescence Transitions in Atomic Fluorescence SpectrometryAimen KhalidNo ratings yet
- B.Eng - Sc. (Mechanical Engineering), Universityof Alexandria, 1970 M.Eng - Sc. (Mechanical Engineering), Universityof Alexandria, 1974Document143 pagesB.Eng - Sc. (Mechanical Engineering), Universityof Alexandria, 1970 M.Eng - Sc. (Mechanical Engineering), Universityof Alexandria, 1974Samik MaitiNo ratings yet
- Blackshear 1956 NACA The Growth of Disturbances in A Flame Generated Shear RegionDocument11 pagesBlackshear 1956 NACA The Growth of Disturbances in A Flame Generated Shear RegionPrem ChandNo ratings yet
- Serial 1949/29:, Inv) ! Stigatlon of Belmhoitz Resouators Report No. B.041Document26 pagesSerial 1949/29:, Inv) ! Stigatlon of Belmhoitz Resouators Report No. B.041AntonioPalloneNo ratings yet
- Mouth State Detection Using Log-Polar SignaturesDocument11 pagesMouth State Detection Using Log-Polar SignaturesMohammad AkifNo ratings yet
- Am. J - Med. and Hyg.,: Communications Enr VolDocument3 pagesAm. J - Med. and Hyg.,: Communications Enr VolFELIPE DANIEL MONTERO BRUNINo ratings yet
- Jresv70cn2p75 A1bDocument14 pagesJresv70cn2p75 A1bZé Pontes DetCordNo ratings yet
- The Effect of Systox On Ionic Fluxes in Excised Barley RootsDocument10 pagesThe Effect of Systox On Ionic Fluxes in Excised Barley RootsSh1vaNo ratings yet
- Et Al. Macaca Fascicularis: 0 1 O0 200 3 O0 I I I 30Document1 pageEt Al. Macaca Fascicularis: 0 1 O0 200 3 O0 I I I 30AkicaNo ratings yet
- R. M. Shapley and C. Enroth-Cugell: Range AnDocument1 pageR. M. Shapley and C. Enroth-Cugell: Range AnAkicaNo ratings yet
- E Lighting: BetweenDocument1 pageE Lighting: BetweenreacharunkNo ratings yet
- bose1997_Preparation_of_nonclassical_states_in_cavities_with_a_movingDocument12 pagesbose1997_Preparation_of_nonclassical_states_in_cavities_with_a_movingVeronika TreumovaNo ratings yet
- Metformin and Sitagliptin Prolonged-Release TabletsDocument7 pagesMetformin and Sitagliptin Prolonged-Release Tabletsmykiend2002No ratings yet
- Night MiopiaDocument8 pagesNight MiopiamurilobsouzaNo ratings yet
- Oth R MetalDocument10 pagesOth R MetalBhavesh ShimpiNo ratings yet
- Medical Division, Air Force Systems Commnand, e 62F Wright-Patterson Air Force Base, Ohio 45433i210-7 78-45 Feb 8Document15 pagesMedical Division, Air Force Systems Commnand, e 62F Wright-Patterson Air Force Base, Ohio 45433i210-7 78-45 Feb 8Bryan396No ratings yet
- Mreznica - 78Document1 pageMreznica - 78AkicaNo ratings yet
- Mreznica - 80Document1 pageMreznica - 80AkicaNo ratings yet
- Mreznica - 79Document1 pageMreznica - 79AkicaNo ratings yet
- Mreznica - 71Document1 pageMreznica - 71AkicaNo ratings yet
- Visual Adaptation and Retinal Gain ControlsDocument1 pageVisual Adaptation and Retinal Gain ControlsAkicaNo ratings yet
- 6.1. Theories of The Retinal Gain Control: C O0 C - ' - ' - I C R oDocument1 page6.1. Theories of The Retinal Gain Control: C O0 C - ' - ' - I C R oAkicaNo ratings yet
- Z,, (Co) Y.L (Co) I, (O ) I, (Co) : Visual Adaptation and Retinal Gain ControlsDocument1 pageZ,, (Co) Y.L (Co) I, (O ) I, (Co) : Visual Adaptation and Retinal Gain ControlsAkicaNo ratings yet
- R. M. Shapley and C. Enroth-Cugell: Et AlDocument1 pageR. M. Shapley and C. Enroth-Cugell: Et AlAkicaNo ratings yet
- Et Al. Macaca Fascicularis: 0 1 O0 200 3 O0 I I I 30Document1 pageEt Al. Macaca Fascicularis: 0 1 O0 200 3 O0 I I I 30AkicaNo ratings yet
- Mreznica - 74Document1 pageMreznica - 74AkicaNo ratings yet
- Visual Adaptation and Retinal Gain Controls Build Up of Particles ( 1 Y2 D Y3 LLLLL) L Y4) 1 Y5 ( Z,)Document1 pageVisual Adaptation and Retinal Gain Controls Build Up of Particles ( 1 Y2 D Y3 LLLLL) L Y4) 1 Y5 ( Z,)AkicaNo ratings yet
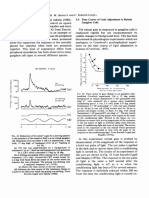
- Time Course of Gain Adjustment in Retinal Ganglion CellsDocument1 pageTime Course of Gain Adjustment in Retinal Ganglion CellsAkicaNo ratings yet
- Mreznica - 66Document1 pageMreznica - 66AkicaNo ratings yet
- Mreznica - 73Document1 pageMreznica - 73AkicaNo ratings yet
- Mreznica - 64Document1 pageMreznica - 64AkicaNo ratings yet
- Do Not: 5. Gain Control in PhotoreceptorsDocument1 pageDo Not: 5. Gain Control in PhotoreceptorsAkicaNo ratings yet
- Tpeak: I - Dark Adapted - 4"4 BG - 3.2 BG - 2.1 BG I I IDocument1 pageTpeak: I - Dark Adapted - 4"4 BG - 3.2 BG - 2.1 BG I I IAkicaNo ratings yet
- Mreznica - 58Document1 pageMreznica - 58AkicaNo ratings yet
- Mreznica - 60Document1 pageMreznica - 60AkicaNo ratings yet
- Mreznica - 61Document1 pageMreznica - 61AkicaNo ratings yet
- Retina - 52Document1 pageRetina - 52AkicaNo ratings yet
- Mreznica - 55Document1 pageMreznica - 55AkicaNo ratings yet
- 4.3. Horizontal Cells: F I I FDocument1 page4.3. Horizontal Cells: F I I FAkicaNo ratings yet
- Visual Adaptation and Retinal Gain ControlsDocument1 pageVisual Adaptation and Retinal Gain ControlsAkicaNo ratings yet
- Retina - 52Document1 pageRetina - 52AkicaNo ratings yet
- Retina - 51Document1 pageRetina - 51AkicaNo ratings yet
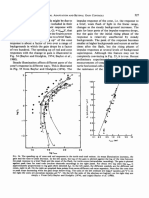
- 3.6. Gain Control and Receptive Field Size Across The Population of Ganglion CellsDocument1 page3.6. Gain Control and Receptive Field Size Across The Population of Ganglion CellsAkicaNo ratings yet
- Retina - 53 PDFDocument1 pageRetina - 53 PDFAkicaNo ratings yet
- Retina - 52Document1 pageRetina - 52AkicaNo ratings yet
- Chicken Foot DissectionDocument2 pagesChicken Foot DissectionGacostaza09No ratings yet
- KS4 Basic Life Support PresentationDocument29 pagesKS4 Basic Life Support PresentationDixiline MarzoNo ratings yet
- Rhodophyta Fact SheetDocument3 pagesRhodophyta Fact Sheetapi-299846771No ratings yet
- Pex 09 03Document4 pagesPex 09 03Marcela Anco Sotomayor50% (4)
- Answer Questions5 1Document3 pagesAnswer Questions5 1yo-chengNo ratings yet
- Khalid Complete Anesthesia Notes PDFDocument361 pagesKhalid Complete Anesthesia Notes PDFDrMuhammad Ishfaq Habib100% (3)
- 2 Quarter Examination S.Y. 2019-2020: Earth Life and Science - Grade 11Document6 pages2 Quarter Examination S.Y. 2019-2020: Earth Life and Science - Grade 11Mihatsu TakiNo ratings yet
- What Is NeurobicsDocument6 pagesWhat Is Neurobicsvarun_009No ratings yet
- Pulsus Paradoxus - Wikip PDFDocument4 pagesPulsus Paradoxus - Wikip PDFAniket MittalNo ratings yet
- Medical Surgical Nursing Exam 19 NLE Style 100 Items Nurseslabs December 2012 NLE ResultsDocument21 pagesMedical Surgical Nursing Exam 19 NLE Style 100 Items Nurseslabs December 2012 NLE ResultsKaren Ann M. Estrada100% (1)
- Fundamentals of Anatomy and Physiology 8Th Edition Martini Test Bank Full Chapter PDFDocument50 pagesFundamentals of Anatomy and Physiology 8Th Edition Martini Test Bank Full Chapter PDFgillanolympia4091253100% (9)
- New Estrogen and ProgesteroneDocument39 pagesNew Estrogen and ProgesteroneWegrimel AriegaraNo ratings yet
- Neonatal Jaundice: Prevention, Assessment and Management 2Document20 pagesNeonatal Jaundice: Prevention, Assessment and Management 2kyawswakyawswaNo ratings yet
- Tratamiento Quirúrgico Del Mal Perforante Plantar: C.M. Hernández-Cañete, J. Borroto-PachecoDocument7 pagesTratamiento Quirúrgico Del Mal Perforante Plantar: C.M. Hernández-Cañete, J. Borroto-PachecoDiegoNo ratings yet
- Physiology Course: Dr. Velu M. RachelDocument40 pagesPhysiology Course: Dr. Velu M. RachelKunda JosephNo ratings yet
- Nursing Care PlanDocument7 pagesNursing Care Planmcd7r883% (6)
- Screenshot 2021-06-23 at 11.36.48 AMDocument116 pagesScreenshot 2021-06-23 at 11.36.48 AMSmitha ShekarNo ratings yet
- Glycine MetabolismDocument28 pagesGlycine MetabolismSuryansh GoyalNo ratings yet
- TRIO For Flute, Guitar, and CelloDocument44 pagesTRIO For Flute, Guitar, and CelloNick Schuller100% (1)
- Unstable Angina With Underlying DyslipidaemiaDocument9 pagesUnstable Angina With Underlying DyslipidaemiaAiman ArifinNo ratings yet
- Plant Body Plant Body: Why Is Histology Important?Document6 pagesPlant Body Plant Body: Why Is Histology Important?ZyreeneNicoleNo ratings yet
- Physical Education Nine Ten English VersionDocument149 pagesPhysical Education Nine Ten English Versiondsafg87g87gawdwNo ratings yet
- Callus CultureDocument24 pagesCallus CultureHabiba Majeed MalikNo ratings yet
- Nursing Process in The Care of The Preterm InfantDocument5 pagesNursing Process in The Care of The Preterm InfantizwanNo ratings yet
- Manage Upper GI Bleed in 33yo Hotel ReceptionistDocument42 pagesManage Upper GI Bleed in 33yo Hotel ReceptionistMuhammad Naveed AslamNo ratings yet
- Psychology Canadian 2nd Edition Ciccarelli Test BankDocument42 pagesPsychology Canadian 2nd Edition Ciccarelli Test BankMichaelAllenrazne100% (10)
- 41 Emotions As Expressed Through Body LanguageDocument9 pages41 Emotions As Expressed Through Body LanguageSocaciu Gabriel100% (1)
- Nursing Care Plan For Postpartum HemorrhageDocument2 pagesNursing Care Plan For Postpartum HemorrhageDianne Mae100% (1)
- Thermoregulation in PiscesDocument4 pagesThermoregulation in PiscesAbellya Nella Morte100% (3)
- All About SharksDocument3 pagesAll About SharksGutmonarchNo ratings yet
- Quantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessFrom EverandQuantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessRating: 4 out of 5 stars4/5 (6)
- A Brief History of Time: From the Big Bang to Black HolesFrom EverandA Brief History of Time: From the Big Bang to Black HolesRating: 4 out of 5 stars4/5 (2193)
- Dark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseFrom EverandDark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseRating: 3.5 out of 5 stars3.5/5 (69)
- Summary and Interpretation of Reality TransurfingFrom EverandSummary and Interpretation of Reality TransurfingRating: 5 out of 5 stars5/5 (5)
- Strange Angel: The Otherworldly Life of Rocket Scientist John Whiteside ParsonsFrom EverandStrange Angel: The Otherworldly Life of Rocket Scientist John Whiteside ParsonsRating: 4 out of 5 stars4/5 (94)
- A Beginner's Guide to Constructing the Universe: The Mathematical Archetypes of Nature, Art, and ScienceFrom EverandA Beginner's Guide to Constructing the Universe: The Mathematical Archetypes of Nature, Art, and ScienceRating: 4 out of 5 stars4/5 (51)
- The Beginning of Infinity: Explanations That Transform the WorldFrom EverandThe Beginning of Infinity: Explanations That Transform the WorldRating: 5 out of 5 stars5/5 (60)
- Packing for Mars: The Curious Science of Life in the VoidFrom EverandPacking for Mars: The Curious Science of Life in the VoidRating: 4 out of 5 stars4/5 (1395)
- Knocking on Heaven's Door: How Physics and Scientific Thinking Illuminate the Universe and the Modern WorldFrom EverandKnocking on Heaven's Door: How Physics and Scientific Thinking Illuminate the Universe and the Modern WorldRating: 3.5 out of 5 stars3.5/5 (64)
- Lost in Math: How Beauty Leads Physics AstrayFrom EverandLost in Math: How Beauty Leads Physics AstrayRating: 4.5 out of 5 stars4.5/5 (125)
- The Tao of Physics: An Exploration of the Parallels between Modern Physics and Eastern MysticismFrom EverandThe Tao of Physics: An Exploration of the Parallels between Modern Physics and Eastern MysticismRating: 4 out of 5 stars4/5 (500)
- The Physics of God: How the Deepest Theories of Science Explain Religion and How the Deepest Truths of Religion Explain ScienceFrom EverandThe Physics of God: How the Deepest Theories of Science Explain Religion and How the Deepest Truths of Religion Explain ScienceRating: 4.5 out of 5 stars4.5/5 (23)
- The End of Everything: (Astrophysically Speaking)From EverandThe End of Everything: (Astrophysically Speaking)Rating: 4.5 out of 5 stars4.5/5 (157)
- Quantum Physics: What Everyone Needs to KnowFrom EverandQuantum Physics: What Everyone Needs to KnowRating: 4.5 out of 5 stars4.5/5 (48)
- Quantum Physics for Beginners Who Flunked Math And Science: Quantum Mechanics And Physics Made Easy Guide In Plain Simple EnglishFrom EverandQuantum Physics for Beginners Who Flunked Math And Science: Quantum Mechanics And Physics Made Easy Guide In Plain Simple EnglishRating: 4.5 out of 5 stars4.5/5 (18)
- Bedeviled: A Shadow History of Demons in ScienceFrom EverandBedeviled: A Shadow History of Demons in ScienceRating: 5 out of 5 stars5/5 (5)
- Midnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterFrom EverandMidnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterRating: 4.5 out of 5 stars4.5/5 (409)
- Black Holes: The Key to Understanding the UniverseFrom EverandBlack Holes: The Key to Understanding the UniverseRating: 4.5 out of 5 stars4.5/5 (13)
- In Search of Schrödinger’s Cat: Quantum Physics and RealityFrom EverandIn Search of Schrödinger’s Cat: Quantum Physics and RealityRating: 4 out of 5 stars4/5 (380)
- The Sounds of Life: How Digital Technology Is Bringing Us Closer to the Worlds of Animals and PlantsFrom EverandThe Sounds of Life: How Digital Technology Is Bringing Us Closer to the Worlds of Animals and PlantsRating: 5 out of 5 stars5/5 (5)
- The Simulated Multiverse: An MIT Computer Scientist Explores Parallel Universes, The Simulation Hypothesis, Quantum Computing and the Mandela EffectFrom EverandThe Simulated Multiverse: An MIT Computer Scientist Explores Parallel Universes, The Simulation Hypothesis, Quantum Computing and the Mandela EffectRating: 4.5 out of 5 stars4.5/5 (20)