Professional Documents
Culture Documents
Thermometric Titration Worksheet
Thermometric Titration Worksheet
Uploaded by
YANGCopyright:
Available Formats
You might also like
- Lab Report Experiment 1 - Rate of Reaction - 2021Document4 pagesLab Report Experiment 1 - Rate of Reaction - 2021Ye Woon LimNo ratings yet
- Test Ten Paper TwoDocument7 pagesTest Ten Paper TwoWanje MichaelNo ratings yet
- Chemistry SPMDocument42 pagesChemistry SPMTeoh Chee TzeNo ratings yet
- Acids, BAIS AND SALTS QDocument10 pagesAcids, BAIS AND SALTS Qexan14431No ratings yet
- Form 3 Mid TRM 1Document4 pagesForm 3 Mid TRM 1Bryan MasikaNo ratings yet
- A Level Chemistry Paper 2 Exam 34Document5 pagesA Level Chemistry Paper 2 Exam 34Anthony AndyNo ratings yet
- 2018 PromosDocument7 pages2018 PromosChloe MontNo ratings yet
- FORM 4 ENERGY CHANGES IN CHEMICAL AND PHYSICAL PROCESSES QUESTIONS Teacher - Co - .KeDocument8 pagesFORM 4 ENERGY CHANGES IN CHEMICAL AND PHYSICAL PROCESSES QUESTIONS Teacher - Co - .KeCitron Akhala100% (1)
- Rates of Reaction o LevelDocument19 pagesRates of Reaction o Leveljosephmadrara2No ratings yet
- Acids, Alkalis and Titrations 1 QPDocument14 pagesAcids, Alkalis and Titrations 1 QPSaad AnsariNo ratings yet
- Chem Pp2 s.6 St. Mary - S Kitende 2020Document5 pagesChem Pp2 s.6 St. Mary - S Kitende 2020nanyonjo shadiaNo ratings yet
- s6 Chemistry Pp2Document5 pagess6 Chemistry Pp2ANYWAR SIMONNo ratings yet
- Date Planned: - / - / - Daily Tutorial Sheet Expected Duration: 90 Min Actual Date of Attempt: - / - / - Level-0 Exact DurationDocument55 pagesDate Planned: - / - / - Daily Tutorial Sheet Expected Duration: 90 Min Actual Date of Attempt: - / - / - Level-0 Exact DurationSickdanNo ratings yet
- New Microsoft Office Word DocumentDocument2 pagesNew Microsoft Office Word DocumentIfhAm HasSanNo ratings yet
- Chemistry P3 0003Document9 pagesChemistry P3 0003Karoki Francis KagombeNo ratings yet
- H2 Chemistry Prelims 2011 (Planning)Document12 pagesH2 Chemistry Prelims 2011 (Planning)iuhihzNo ratings yet
- Chem Sat PracDocument5 pagesChem Sat PracndirangunancynjokiNo ratings yet
- Acids, Alkalis and Titrations and Paper 1Document26 pagesAcids, Alkalis and Titrations and Paper 1DinangaNo ratings yet
- Practice Questions On CHM 212Document4 pagesPractice Questions On CHM 212Help HandNo ratings yet
- Thermometric TitrationDocument3 pagesThermometric TitrationRangerNo ratings yet
- Rate of Reaction Part 1Document3 pagesRate of Reaction Part 1Subesh ShanmugamNo ratings yet
- Physical Chemistry QuestionsDocument22 pagesPhysical Chemistry QuestionshanaNo ratings yet
- Che 320 ExamDocument3 pagesChe 320 ExamAnjolaoluwa Oreoluwa AfolabiNo ratings yet
- Chem - Rates of Reaction ProblemsDocument6 pagesChem - Rates of Reaction ProblemslyagobaNo ratings yet
- How FastDocument54 pagesHow FastKaushal Silva RanpatabendigeNo ratings yet
- As MolesDocument5 pagesAs MolesHaider AliNo ratings yet
- Revision STPM Term 1Document15 pagesRevision STPM Term 1Wong WengSiongNo ratings yet
- SPM Kimia Tingkatan, 5 Rate of Reaction ExerciseDocument7 pagesSPM Kimia Tingkatan, 5 Rate of Reaction Exerciseryder1man6433No ratings yet
- Unit 4 ExamDocument20 pagesUnit 4 ExamRohini SelvarajahNo ratings yet
- Acids and Bases 2Document35 pagesAcids and Bases 24D-31 WONG YUEN TSZNo ratings yet
- Acids, Alkalis and Titrations Paper 2Document15 pagesAcids, Alkalis and Titrations Paper 2DinangaNo ratings yet
- SCH 101HFN 141 Introduction To Physical ChemistryDocument4 pagesSCH 101HFN 141 Introduction To Physical Chemistryodib478No ratings yet
- Unit 1 Manual 2019Document18 pagesUnit 1 Manual 2019JozelleNo ratings yet
- Chemical Energetics Unit3 FCDocument31 pagesChemical Energetics Unit3 FCIbrahim AbidNo ratings yet
- Chemistry Practical Exam 5 QuestionsDocument12 pagesChemistry Practical Exam 5 Questionskotogboehenry3No ratings yet
- C8 Chemistry Rates and Equilibrium HomeworkDocument8 pagesC8 Chemistry Rates and Equilibrium HomeworkChloeYapYanQiNo ratings yet
- Exercise Chemistry 1Document10 pagesExercise Chemistry 1Sarah LeeNo ratings yet
- Practice FRQDocument4 pagesPractice FRQKrystal LiNo ratings yet
- Samp 103151 Exam V2 S210Document15 pagesSamp 103151 Exam V2 S210gpeck92No ratings yet
- Test 2 For A2Document15 pagesTest 2 For A2gkawsar22No ratings yet
- 2000 - RD - 1 - Questions - tcm18-190754Document9 pages2000 - RD - 1 - Questions - tcm18-190754LouiseflemingNo ratings yet
- PS 5.1.2 Enthalpy CalculationsDocument3 pagesPS 5.1.2 Enthalpy Calculationsrichard.gross62No ratings yet
- AP Chemistry 1998 Free ResponseDocument7 pagesAP Chemistry 1998 Free Responsesabbate1994No ratings yet
- Chemistry Practical Exam 8 QuestionsDocument8 pagesChemistry Practical Exam 8 Questionsgiftonnakholi500No ratings yet
- Chemistry2A F4 2023Document3 pagesChemistry2A F4 2023aishanassor624No ratings yet
- Chemistry Practical Exam 40 QuestionsDocument8 pagesChemistry Practical Exam 40 Questionskotogboehenry3No ratings yet
- Chemistry 1, Fosce 2024 2Document4 pagesChemistry 1, Fosce 2024 2elishamahubiNo ratings yet
- Chapter 1: Rate of Reaction: Larning Task 1.2 Problem SolvingDocument29 pagesChapter 1: Rate of Reaction: Larning Task 1.2 Problem Solvingamin_zamanNo ratings yet
- Chem 3Document6 pagesChem 3githukucharles.gcNo ratings yet
- 2014-2015 Equilibrium Free Response - SolutionsDocument4 pages2014-2015 Equilibrium Free Response - SolutionsAniket TiwariNo ratings yet
- Chemistry PracticalDocument3 pagesChemistry PracticalNassrah JumaNo ratings yet
- Year 10 Chemistry - Practice Exam Questions Rates and REversible ReactionsDocument4 pagesYear 10 Chemistry - Practice Exam Questions Rates and REversible ReactionsAshleyn Mary SandersNo ratings yet
- A Level Chemistry Paper 2 Exam 26Document5 pagesA Level Chemistry Paper 2 Exam 26Anthony AndyNo ratings yet
- AP Chemistry 1994 Free ResponseDocument5 pagesAP Chemistry 1994 Free ResponseWes BristleconeNo ratings yet
- A Level CHEM ZOOM SEMINAR4Document5 pagesA Level CHEM ZOOM SEMINAR4Drake AmbroseNo ratings yet
- (SAT) Chemistry Sample Paper 11Document4 pages(SAT) Chemistry Sample Paper 11Aman9692No ratings yet
- ALEVELREVISIONQUESTIONSDocument7 pagesALEVELREVISIONQUESTIONSAnthony AndyNo ratings yet
- Chem G-9 Lesson 7 IGCSE Qs - Rates of ReactionDocument24 pagesChem G-9 Lesson 7 IGCSE Qs - Rates of ReactionKarim WaelNo ratings yet
- Chemical Eq. R C MukarjeeDocument48 pagesChemical Eq. R C MukarjeevaibhavNo ratings yet
Thermometric Titration Worksheet
Thermometric Titration Worksheet
Uploaded by
YANGOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Thermometric Titration Worksheet
Thermometric Titration Worksheet
Uploaded by
YANGCopyright:
Available Formats
Thermometric Titration Worksheet
January 2011
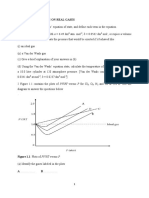
1. It is possible to determine the end point of an acid-base reaction by measuring
the temperature changes when different volumes of a strong acid react with a
strong base. Table 1 shows the volumes of sulphuric acid used to react with 25
cm3 of 2.0 moldm-3 sodium hydroxide and the temperature changes that
occurred. Figure 1 shows the thermometer readings for the addition of 10 cm 3
and 20 cm3 of the H2SO4.
(a) (i) Differentiate between a ‘strong acid’ and a ‘weak acid’. (2 marks)
(ii) Suggest ONE other base that could be used instead of NaOH.
(1
mark)
(iii) Using the thermometer readings in Figure 1, complete Table 1 by
recording the temperature for the addition of 10 cm3 and 20 cm3 of
H2SO4. (2 marks)
(iv) Using a graph paper, plot the points of temperature against volume
of acid added. (3 marks)
(v) Draw the TWO lines of best fit through the points in (iv) above
where the temperature is increasing and where the temperature is
decreasing and hence determine the end point of the reaction.
(3
marks)
(vi) Write a balanced equation for the reaction. (2 marks)
(vii) Calculate the concentration of H2SO4 in moldm-3.(2 marks)
Total 15 marks
You might also like
- Lab Report Experiment 1 - Rate of Reaction - 2021Document4 pagesLab Report Experiment 1 - Rate of Reaction - 2021Ye Woon LimNo ratings yet
- Test Ten Paper TwoDocument7 pagesTest Ten Paper TwoWanje MichaelNo ratings yet
- Chemistry SPMDocument42 pagesChemistry SPMTeoh Chee TzeNo ratings yet
- Acids, BAIS AND SALTS QDocument10 pagesAcids, BAIS AND SALTS Qexan14431No ratings yet
- Form 3 Mid TRM 1Document4 pagesForm 3 Mid TRM 1Bryan MasikaNo ratings yet
- A Level Chemistry Paper 2 Exam 34Document5 pagesA Level Chemistry Paper 2 Exam 34Anthony AndyNo ratings yet
- 2018 PromosDocument7 pages2018 PromosChloe MontNo ratings yet
- FORM 4 ENERGY CHANGES IN CHEMICAL AND PHYSICAL PROCESSES QUESTIONS Teacher - Co - .KeDocument8 pagesFORM 4 ENERGY CHANGES IN CHEMICAL AND PHYSICAL PROCESSES QUESTIONS Teacher - Co - .KeCitron Akhala100% (1)
- Rates of Reaction o LevelDocument19 pagesRates of Reaction o Leveljosephmadrara2No ratings yet
- Acids, Alkalis and Titrations 1 QPDocument14 pagesAcids, Alkalis and Titrations 1 QPSaad AnsariNo ratings yet
- Chem Pp2 s.6 St. Mary - S Kitende 2020Document5 pagesChem Pp2 s.6 St. Mary - S Kitende 2020nanyonjo shadiaNo ratings yet
- s6 Chemistry Pp2Document5 pagess6 Chemistry Pp2ANYWAR SIMONNo ratings yet
- Date Planned: - / - / - Daily Tutorial Sheet Expected Duration: 90 Min Actual Date of Attempt: - / - / - Level-0 Exact DurationDocument55 pagesDate Planned: - / - / - Daily Tutorial Sheet Expected Duration: 90 Min Actual Date of Attempt: - / - / - Level-0 Exact DurationSickdanNo ratings yet
- New Microsoft Office Word DocumentDocument2 pagesNew Microsoft Office Word DocumentIfhAm HasSanNo ratings yet
- Chemistry P3 0003Document9 pagesChemistry P3 0003Karoki Francis KagombeNo ratings yet
- H2 Chemistry Prelims 2011 (Planning)Document12 pagesH2 Chemistry Prelims 2011 (Planning)iuhihzNo ratings yet
- Chem Sat PracDocument5 pagesChem Sat PracndirangunancynjokiNo ratings yet
- Acids, Alkalis and Titrations and Paper 1Document26 pagesAcids, Alkalis and Titrations and Paper 1DinangaNo ratings yet
- Practice Questions On CHM 212Document4 pagesPractice Questions On CHM 212Help HandNo ratings yet
- Thermometric TitrationDocument3 pagesThermometric TitrationRangerNo ratings yet
- Rate of Reaction Part 1Document3 pagesRate of Reaction Part 1Subesh ShanmugamNo ratings yet
- Physical Chemistry QuestionsDocument22 pagesPhysical Chemistry QuestionshanaNo ratings yet
- Che 320 ExamDocument3 pagesChe 320 ExamAnjolaoluwa Oreoluwa AfolabiNo ratings yet
- Chem - Rates of Reaction ProblemsDocument6 pagesChem - Rates of Reaction ProblemslyagobaNo ratings yet
- How FastDocument54 pagesHow FastKaushal Silva RanpatabendigeNo ratings yet
- As MolesDocument5 pagesAs MolesHaider AliNo ratings yet
- Revision STPM Term 1Document15 pagesRevision STPM Term 1Wong WengSiongNo ratings yet
- SPM Kimia Tingkatan, 5 Rate of Reaction ExerciseDocument7 pagesSPM Kimia Tingkatan, 5 Rate of Reaction Exerciseryder1man6433No ratings yet
- Unit 4 ExamDocument20 pagesUnit 4 ExamRohini SelvarajahNo ratings yet
- Acids and Bases 2Document35 pagesAcids and Bases 24D-31 WONG YUEN TSZNo ratings yet
- Acids, Alkalis and Titrations Paper 2Document15 pagesAcids, Alkalis and Titrations Paper 2DinangaNo ratings yet
- SCH 101HFN 141 Introduction To Physical ChemistryDocument4 pagesSCH 101HFN 141 Introduction To Physical Chemistryodib478No ratings yet
- Unit 1 Manual 2019Document18 pagesUnit 1 Manual 2019JozelleNo ratings yet
- Chemical Energetics Unit3 FCDocument31 pagesChemical Energetics Unit3 FCIbrahim AbidNo ratings yet
- Chemistry Practical Exam 5 QuestionsDocument12 pagesChemistry Practical Exam 5 Questionskotogboehenry3No ratings yet
- C8 Chemistry Rates and Equilibrium HomeworkDocument8 pagesC8 Chemistry Rates and Equilibrium HomeworkChloeYapYanQiNo ratings yet
- Exercise Chemistry 1Document10 pagesExercise Chemistry 1Sarah LeeNo ratings yet
- Practice FRQDocument4 pagesPractice FRQKrystal LiNo ratings yet
- Samp 103151 Exam V2 S210Document15 pagesSamp 103151 Exam V2 S210gpeck92No ratings yet
- Test 2 For A2Document15 pagesTest 2 For A2gkawsar22No ratings yet
- 2000 - RD - 1 - Questions - tcm18-190754Document9 pages2000 - RD - 1 - Questions - tcm18-190754LouiseflemingNo ratings yet
- PS 5.1.2 Enthalpy CalculationsDocument3 pagesPS 5.1.2 Enthalpy Calculationsrichard.gross62No ratings yet
- AP Chemistry 1998 Free ResponseDocument7 pagesAP Chemistry 1998 Free Responsesabbate1994No ratings yet
- Chemistry Practical Exam 8 QuestionsDocument8 pagesChemistry Practical Exam 8 Questionsgiftonnakholi500No ratings yet
- Chemistry2A F4 2023Document3 pagesChemistry2A F4 2023aishanassor624No ratings yet
- Chemistry Practical Exam 40 QuestionsDocument8 pagesChemistry Practical Exam 40 Questionskotogboehenry3No ratings yet
- Chemistry 1, Fosce 2024 2Document4 pagesChemistry 1, Fosce 2024 2elishamahubiNo ratings yet
- Chapter 1: Rate of Reaction: Larning Task 1.2 Problem SolvingDocument29 pagesChapter 1: Rate of Reaction: Larning Task 1.2 Problem Solvingamin_zamanNo ratings yet
- Chem 3Document6 pagesChem 3githukucharles.gcNo ratings yet
- 2014-2015 Equilibrium Free Response - SolutionsDocument4 pages2014-2015 Equilibrium Free Response - SolutionsAniket TiwariNo ratings yet
- Chemistry PracticalDocument3 pagesChemistry PracticalNassrah JumaNo ratings yet
- Year 10 Chemistry - Practice Exam Questions Rates and REversible ReactionsDocument4 pagesYear 10 Chemistry - Practice Exam Questions Rates and REversible ReactionsAshleyn Mary SandersNo ratings yet
- A Level Chemistry Paper 2 Exam 26Document5 pagesA Level Chemistry Paper 2 Exam 26Anthony AndyNo ratings yet
- AP Chemistry 1994 Free ResponseDocument5 pagesAP Chemistry 1994 Free ResponseWes BristleconeNo ratings yet
- A Level CHEM ZOOM SEMINAR4Document5 pagesA Level CHEM ZOOM SEMINAR4Drake AmbroseNo ratings yet
- (SAT) Chemistry Sample Paper 11Document4 pages(SAT) Chemistry Sample Paper 11Aman9692No ratings yet
- ALEVELREVISIONQUESTIONSDocument7 pagesALEVELREVISIONQUESTIONSAnthony AndyNo ratings yet
- Chem G-9 Lesson 7 IGCSE Qs - Rates of ReactionDocument24 pagesChem G-9 Lesson 7 IGCSE Qs - Rates of ReactionKarim WaelNo ratings yet
- Chemical Eq. R C MukarjeeDocument48 pagesChemical Eq. R C MukarjeevaibhavNo ratings yet