Professional Documents
Culture Documents
Analysis
Uploaded by
Oliver Tabag0 ratings0% found this document useful (0 votes)
63 views4 pagesThe report summarizes the results of a forced spirometry test conducted on a 21-year old male patient as part of a clinical trial. The initial test results were deemed unacceptable but met acceptability criteria after statistical review. Specifically, the patient's FEV1 was acceptable but his FVC was underestimated since he could not exhale for longer than 6 seconds. After revisions to the quality control statements and grades, the data was determined to meet criteria for acceptability and the patient may be eligible for randomization in the clinical trial.
Original Description:
v1
Original Title
analysis
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe report summarizes the results of a forced spirometry test conducted on a 21-year old male patient as part of a clinical trial. The initial test results were deemed unacceptable but met acceptability criteria after statistical review. Specifically, the patient's FEV1 was acceptable but his FVC was underestimated since he could not exhale for longer than 6 seconds. After revisions to the quality control statements and grades, the data was determined to meet criteria for acceptability and the patient may be eligible for randomization in the clinical trial.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
63 views4 pagesAnalysis
Uploaded by
Oliver TabagThe report summarizes the results of a forced spirometry test conducted on a 21-year old male patient as part of a clinical trial. The initial test results were deemed unacceptable but met acceptability criteria after statistical review. Specifically, the patient's FEV1 was acceptable but his FVC was underestimated since he could not exhale for longer than 6 seconds. After revisions to the quality control statements and grades, the data was determined to meet criteria for acceptability and the patient may be eligible for randomization in the clinical trial.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 4
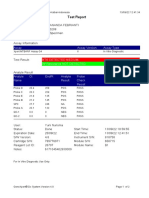
PFT Analysis Report (Revised 13-Jul-2018)
Sponsor: Novartis Protocol: CQAW039A2315 ERT Account: 800387
Investigator: Atienza, Tito Sponsor Site ID: 3553 Measurement Type: Forced Spirometry
Patient ID: 3553002 SYS#: 800387-040928-000007
Visit: V1 Timepoint: PRE PFT Transmittal Number: 42134303
Ethnicity: South East Asian Sex: Male Height: 155.0cm
Age: 21.00
Collection Date: 06-Jul-2018 Collection Time: 07:48:42 Collected by: Tabag, Oliver
Original Data
QC Statements:
[Forced Spirometry All Efforts] Data meets ATS/ERS criteria for acceptable quality; patient might be randomized. Please check
other screening visit PFT data for quality messages and observe study inclusion/exclusion criteria.
[Forced Spirometry Best FEV1] Subject did not exhale for longer than 6 seconds.
[Forced Spirometry QC Grade] Acceptable Forced Spirometry measurement received.
Parameter Pred 1st 2nd 3rd 4th 5th 6th 7th 8th Best %Pred
Trial Time 07:49:18 07:50:41 07:52:08 07:53:36
FEV1 [L] 3.152 2.041 1.989 *2.057 2.047 2.057 65
FVC [L] 3.524 2.481 2.481 *2.509 2.484 2.509 71
FEV1/FVC 0.898 0.823 0.802 0.820 0.824 0.820 91
Error Codes D D
Operator Comment: acceptable
After BTR
BTR Statements:
[Forced Spirometry BTR] Forced Spirometry measurement unchanged.
[Forced Spirometry BTR] Data meets ATS/ERS criteria for acceptable quality; patient might be randomized. Please check other
screening visit PFT data for quality messages and observe study inclusion/exclusion criteria.
[Comments Acceptable Grade after BTR] FEV1 is acceptable however FVC is underestimated (Technician must enter a comment
and FEV1 must be valid in order for the effort to be considered Acceptable).
[Forced Spirometry BTR Grade] Acceptable Forced Spirometry measurement after BTR.
[OR Note] Grades and comments revised per email from site "patient cannot exhale longer"
[OR Note] Please remember to always provide a comment when the subject cannot exhale longer than 6 seconds
Parameter Pred 1st 2nd 3rd 4th 5th 6th 7th 8th Best %Pred
Trial Time 07:49:18 07:50:41 07:52:08 07:53:36
FEV1 [L] 3.152 2.041 1.989 *2.057 2.047 2.057 65
FVC [L] 3.524 2.481 2.481 *2.509 2.484 2.509 71
FEV1/FVC 0.898 0.823 0.802 0.820 0.824 0.820 91
Error Codes D D
OverReader: Brissenden, Debbie Date: 06-JUL-2018 15:02:08 EDT -04:00
QC By: Austin, Carrie Date: 13-JUL-2018 17:25:03 EDT -04:00
Error Codes:
A No repeatability; Less than 3 accepted forced measurements E No plateau was detected at the end of the expiration
B FEV1 repeatability is unacceptable F Back extrapolation volume was too large
C FVC repeatability is unacceptable H Late peak flow detected
D Expiration time was too short I Coughing was detected in the first part of the expiration
The following analysis information was modified from the previous report:
QA Statement Added: [Forced Spirometry All Efforts] Data meets ATS/ERS criteria for acceptable quality; patient might be
randomized. Please check other screening visit PFT data for quality messages and observe study inclusion/exclusion criteria.
QA Statement Added: [Forced Spirometry QC Grade] Acceptable Forced Spirometry measurement received.
QA Statement Deleted: [Forced Spirometry All Efforts] Patient CANNOT be randomized, because data does not meet the
ATS/ERS criteria for acceptable quality PFT data at screening visit.
QA Statement Deleted: [Forced Spirometry QC Grade] Unacceptable Forced Spirometry measurement received.
BTR Statement Deleted: [Forced Spirometry BTR] Patient CANNOT be randomized, because data does not meet the ATS/ERS
criteria for acceptable quality PFT data at screening visit.
BTR Statement Added: [Forced Spirometry BTR] Data meets ATS/ERS criteria for acceptable quality; patient might be
randomized. Please check other screening visit PFT data for quality messages and observe study inclusion/exclusion criteria.
BTR Statement Added: [Comments Acceptable Grade after BTR] FEV1 is acceptable however FVC is underestimated
(Technician must enter a comment and FEV1 must be valid in order for the effort to be considered Acceptable).
BTR Statement Deleted: [Comments Unacceptable Grade after BTR] Subject did not exhale for longer than 6 seconds.
BTR Statement Deleted: [Forced Spirometry BTR Grade] Unacceptable Forced Spirometry measurement after BTR.
BTR Statement Added: [Forced Spirometry BTR Grade] Acceptable Forced Spirometry measurement after BTR.
BTR Statement Added: Grades and comments revised per email from site "patient cannot exhale longer"
BTR Statement Added: Please remember to always provide a comment when the subject cannot exhale longer than 6
seconds
BTR Statement Deleted: please provide a comment subject cannot exhale any longer
QC By: Taylor, Colleen Date: 08-JUL-2018 22:58:20 EDT -04:00
Generated on: 13-JUL-2018 17:30:08 EDT -04:00 Page 1 of 4
PFT Analysis Report (Revised 13-Jul-2018)
Sponsor: Novartis Protocol: CQAW039A2315 ERT Account: 800387
Investigator: Atienza, Tito Sponsor Site ID: 3553 Measurement Type: Forced Spirometry
Patient ID: 3553002 SYS#: 800387-040928-000007
Visit: V1 Timepoint: PRE PFT Transmittal Number: 42134303
Ethnicity: South East Asian Sex: Male Height: 155.0cm
Age: 21.00
Collection Date: 06-Jul-2018 Collection Time: 07:48:42 Collected by: Tabag, Oliver
For Site Use Only: Please sign and date and file with your source documents.
_________________________________________ ___________________________________________ _________________________
Printed Name of Investigator Signature Date
Comments
Generated on: 13-JUL-2018 17:30:08 EDT -04:00 Page 2 of 4
PFT Analysis Report (Revised 13-Jul-2018)
Sponsor: Novartis Protocol: CQAW039A2315 ERT Account: 800387
Investigator: Atienza, Tito Sponsor Site ID: 3553 Measurement Type: Forced Spirometry
Patient ID: 3553002 SYS#: 800387-040928-000007
Visit: V1 Timepoint: PRE PFT Transmittal Number: 42134303
Ethnicity: South East Asian Sex: Male Height: 155.0cm
Age: 21.00
Collection Date: 06-Jul-2018 Collection Time: 07:48:42 Collected by: Tabag, Oliver
Original Original
After BTR After BTR
Generated on: 13-JUL-2018 17:30:08 EDT -04:00 Page 3 of 4
PFT Analysis Report (Revised 13-Jul-2018)
Sponsor: Novartis Protocol: CQAW039A2315 ERT Account: 800387
Investigator: Atienza, Tito Sponsor Site ID: 3553 Measurement Type: Forced Spirometry
Patient ID: 3553002 SYS#: 800387-040928-000007
Visit: V1 Timepoint: PRE PFT Transmittal Number: 42134303
Ethnicity: South East Asian Sex: Male Height: 155.0cm
Age: 21.00
Collection Date: 06-Jul-2018 Collection Time: 07:48:42 Collected by: Tabag, Oliver
Original
After BTR
Generated on: 13-JUL-2018 17:30:08 EDT -04:00 Page 4 of 4
You might also like
- Credit Card Account 4055 9920 4753 0161Document2 pagesCredit Card Account 4055 9920 4753 0161Oliver TabagNo ratings yet
- Max Lab ReportDocument1 pageMax Lab ReportKallu PrasadNo ratings yet
- Patient Details Specimen Details Physician DetailsDocument1 pagePatient Details Specimen Details Physician DetailsMax WellsNo ratings yet
- Mitra Sambamurthy 2011Document13 pagesMitra Sambamurthy 2011Niky Triantafilo NuñezNo ratings yet
- (Michigan Teacher Training) Jerry G. Gebhard-Teaching English As A Foreign or Second Language - A Teacher Self-Development and Methodology Guide-The University of Michigan Press (2013)Document9 pages(Michigan Teacher Training) Jerry G. Gebhard-Teaching English As A Foreign or Second Language - A Teacher Self-Development and Methodology Guide-The University of Michigan Press (2013)eva17% (6)
- My Helper Report PDFDocument29 pagesMy Helper Report PDFHimanshu Negi100% (3)
- Kyle CVDocument3 pagesKyle CVKyle ByrneNo ratings yet
- Registration Form Proficiency Testing Program: Name Designation Mob. / Tel. NoDocument4 pagesRegistration Form Proficiency Testing Program: Name Designation Mob. / Tel. NoR.K. CONSULTANCY AND CONTRACTORNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay TypesetyawanankesNo ratings yet
- BilirubinDocument1 pageBilirubinAziz Bin Josim100% (1)
- MountainShilajitresin Lab Reports 2019 SpectroDocument3 pagesMountainShilajitresin Lab Reports 2019 SpectroMayur PatelNo ratings yet
- D-37/1, TTC MIDC, Turbhe, Navi Mumbai-400 703: Name Ref. by Test Asked::: Patientid: Home CollectionDocument3 pagesD-37/1, TTC MIDC, Turbhe, Navi Mumbai-400 703: Name Ref. by Test Asked::: Patientid: Home CollectionparentingwithjignaNo ratings yet
- Interpretation: L55 - PSC Indra Nagar Home Visit GRD FLR 197/A, Indra Nagar, Double ROAD, BANGALORE - 560080 BangaloreDocument2 pagesInterpretation: L55 - PSC Indra Nagar Home Visit GRD FLR 197/A, Indra Nagar, Double ROAD, BANGALORE - 560080 BangaloreDivyaSNo ratings yet
- LPLT12358 : Clinical UseDocument2 pagesLPLT12358 : Clinical UseKishor SinghNo ratings yet
- Plot No.428, Phase-IV, Udyog Vihar, Gurgaon, Haryana - 122 015Document3 pagesPlot No.428, Phase-IV, Udyog Vihar, Gurgaon, Haryana - 122 015Asit ANo ratings yet
- BAN63C25302981625499Document3 pagesBAN63C25302981625499sanjeevbiradar121No ratings yet
- Registration Form Paver Block 114 2022Document4 pagesRegistration Form Paver Block 114 2022nagarjunareddyNo ratings yet
- ANEXO7Document14 pagesANEXO7liuming farfanNo ratings yet
- 5dd83a85c39cd 2te6rg0fDocument2 pages5dd83a85c39cd 2te6rg0fDiego AlfonsoNo ratings yet
- This Is An Electronic Report & Not: To Be Used For Any Legal PurposesDocument1 pageThis Is An Electronic Report & Not: To Be Used For Any Legal PurposesTauseef Taj KianiNo ratings yet
- P.T. Margacipta Wiragrya: (Electric / Trafo Type)Document1 pageP.T. Margacipta Wiragrya: (Electric / Trafo Type)diah kartikaNo ratings yet
- MIDC Hall Boisar Road Thane - 401501 Hba, Scre, TSH Self Swati Patil (40Y/F)Document5 pagesMIDC Hall Boisar Road Thane - 401501 Hba, Scre, TSH Self Swati Patil (40Y/F)TANUNo ratings yet
- Plot No.428, Phase-IV, Udyog Vihar, Gurgaon, Haryana - 122 015Document3 pagesPlot No.428, Phase-IV, Udyog Vihar, Gurgaon, Haryana - 122 015Ritwikk ChakrabortyNo ratings yet
- Diptajyoti Mitra ReportsDocument2 pagesDiptajyoti Mitra ReportsBuddhadeb ChatterjeeNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay TypeMelania Dessy Savitri SunarjadiNo ratings yet
- Jeewan Jyoti Lab PM Integra ReportDocument2 pagesJeewan Jyoti Lab PM Integra Reportpranshu126545No ratings yet
- Doc2 PDFDocument1 pageDoc2 PDFKaty CobbNo ratings yet
- M.SAIDI 2023.09.13 09.58.13 DetailsDocument2 pagesM.SAIDI 2023.09.13 09.58.13 DetailsLaboratorium RSI Sultan Agung BanjarbaruNo ratings yet
- Printable Version - Approval Reference No - 60247630Document2 pagesPrintable Version - Approval Reference No - 60247630sohaibkhaled5858No ratings yet
- Lalpath Shruti ThrroidDocument2 pagesLalpath Shruti ThrroidSatish SrivastavaNo ratings yet
- Plot No.428, Phase-IV, Udyog Vihar, Gurgaon, Haryana - 122 015Document3 pagesPlot No.428, Phase-IV, Udyog Vihar, Gurgaon, Haryana - 122 015Tanuruchi SahaNo ratings yet
- 3Document6 pages3Jose F. Ramirez MendozaNo ratings yet
- Ananda FebriantiDocument2 pagesAnanda FebriantiInternis RsuyarsiNo ratings yet
- Test Report: The Following Sample(s) Was/were Submitted and Identified By/on Behalf of The Client AsDocument4 pagesTest Report: The Following Sample(s) Was/were Submitted and Identified By/on Behalf of The Client Assensor_versionNo ratings yet
- Medical DiagnosisDocument1 pageMedical Diagnosishh servicesNo ratings yet
- Evi Anus ChaniagoDocument11 pagesEvi Anus Chaniagorode sihombingNo ratings yet
- Timson Elijah Oluwasegun - Ogitech 2023 - 2024 Registration SlipDocument1 pageTimson Elijah Oluwasegun - Ogitech 2023 - 2024 Registration Sliptimson segunNo ratings yet
- COA Report CitrullineDocument1 pageCOA Report Citrullinevimal8No ratings yet
- RTCPR Certificate YUVRAJDocument3 pagesRTCPR Certificate YUVRAJRajat SharmaNo ratings yet
- D-37/1, TTC MIDC, Turbhe, Navi Mumbai-400 703: Processed At: ThyrocareDocument3 pagesD-37/1, TTC MIDC, Turbhe, Navi Mumbai-400 703: Processed At: ThyrocareNaitik N ShahNo ratings yet
- BAN63C25302981625435Document3 pagesBAN63C25302981625435sanjeevbiradar121No ratings yet
- Ilabcorp: Tiggs, SamariaDocument1 pageIlabcorp: Tiggs, SamariaSamaria TiggsNo ratings yet
- Plot No.428, Phase-IV, Udyog Vihar, Gurgaon, Haryana - 122 015Document3 pagesPlot No.428, Phase-IV, Udyog Vihar, Gurgaon, Haryana - 122 015Asit ANo ratings yet
- Plot No.428, Phase-IV, Udyog Vihar, Gurgaon, Haryana - 122 015Document3 pagesPlot No.428, Phase-IV, Udyog Vihar, Gurgaon, Haryana - 122 015aditya bNo ratings yet
- Ashu YadavDocument1 pageAshu YadavBhawani SinghNo ratings yet
- STRC 21010785 NDocument1 pageSTRC 21010785 NDhirendra TopalNo ratings yet
- DR Lal Pathlabs: LPL - Lpl-Rohini (National Reference Lab) Sector - 18, Block - E Rohini Delhi 110085Document2 pagesDR Lal Pathlabs: LPL - Lpl-Rohini (National Reference Lab) Sector - 18, Block - E Rohini Delhi 110085Vivek PatelNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay Typeyossy aprillyaNo ratings yet
- 2023.05.06 08.22.15 DetailsDocument2 pages2023.05.06 08.22.15 Detailsakreditasi tarogong 2023No ratings yet
- Interpretation: LPL - Production Test Collection Centre Sector - 18, Block-E Rohini DELHI 110085Document2 pagesInterpretation: LPL - Production Test Collection Centre Sector - 18, Block-E Rohini DELHI 110085masoom rajaNo ratings yet
- TN039C56226475386137 RLSDocument3 pagesTN039C56226475386137 RLSnithya nithya0% (1)
- Max Lab ReportDocument8 pagesMax Lab ReportKallu PrasadNo ratings yet
- LPL - Lpl-Rohini (National Reference Lab) Sector - 18, Block - E Rohini DELHI 110085Document2 pagesLPL - Lpl-Rohini (National Reference Lab) Sector - 18, Block - E Rohini DELHI 110085Ss LaptopNo ratings yet
- Sky - Ibrahim SabryDocument1 pageSky - Ibrahim SabryIbrahim Sabry RehabNo ratings yet
- PT Philips Indonesia Commercial Customer Service Report: Address: Contact InformationDocument2 pagesPT Philips Indonesia Commercial Customer Service Report: Address: Contact InformationYuda BharataNo ratings yet
- FHC ChittarikkalDocument1 pageFHC Chittarikkalkevin princeNo ratings yet
- Chouhatta, Opp Darbhanga House, Ashok Rajpath RD, Patna-800 004Document3 pagesChouhatta, Opp Darbhanga House, Ashok Rajpath RD, Patna-800 004Amarjeet SinhaNo ratings yet
- Covid-19 Test Report: Chha Sgarh Ins Tute of Medical Sciences, Bilaspur, Chha SgarhDocument1 pageCovid-19 Test Report: Chha Sgarh Ins Tute of Medical Sciences, Bilaspur, Chha SgarhAbhijit YadavNo ratings yet
- Plot No.428, Phase-IV, Udyog Vihar, Gurgaon, Haryana - 122 015Document3 pagesPlot No.428, Phase-IV, Udyog Vihar, Gurgaon, Haryana - 122 015Chhotu JhaNo ratings yet
- D-37/1, TTC MIDC, Turbhe, Navi Mumbai-400 703: ThyrocareDocument3 pagesD-37/1, TTC MIDC, Turbhe, Navi Mumbai-400 703: ThyrocareSuraj IngaleNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument6 pagesTest Report: Assay Assay Version Assay TypeJose F. Ramirez MendozaNo ratings yet
- Eviie@: On:T0SmmDocument1 pageEviie@: On:T0SmmK P Vignesh RaoNo ratings yet
- USA - BioFire - EC - 01232023Document4 pagesUSA - BioFire - EC - 01232023yousrazeidan1979No ratings yet
- Gaurav Pathak Thyroxine Free Baf1982f Ddb7 4d20 8c6f 3c95a3649986Document4 pagesGaurav Pathak Thyroxine Free Baf1982f Ddb7 4d20 8c6f 3c95a3649986joshi.shobha88No ratings yet
- Ligaya: Ultraelectromagneticpop!Document8 pagesLigaya: Ultraelectromagneticpop!Oliver TabagNo ratings yet
- Diy Lte YagiDocument18 pagesDiy Lte YagiOliver TabagNo ratings yet
- Vertis 2Document2 pagesVertis 2Oliver TabagNo ratings yet
- August 2018 Monday Tuesday Wednesday Thursday Friday Saturday Sunday 30 31 01 02 03 04 05Document1 pageAugust 2018 Monday Tuesday Wednesday Thursday Friday Saturday Sunday 30 31 01 02 03 04 05Oliver TabagNo ratings yet
- February 2018 Monday Tuesday Wednesday Thursday Friday Saturday Sunday 29 30 31 01 02 03 04Document1 pageFebruary 2018 Monday Tuesday Wednesday Thursday Friday Saturday Sunday 29 30 31 01 02 03 04Oliver TabagNo ratings yet
- SeptDocument1 pageSeptOliver TabagNo ratings yet
- JulyDocument1 pageJulyOliver TabagNo ratings yet
- PCCPDocument1 pagePCCPOliver TabagNo ratings yet
- TotilacDocument4 pagesTotilacOliver Tabag100% (2)
- Fast Facts On The Philippine PassportDocument19 pagesFast Facts On The Philippine PassportOliver TabagNo ratings yet
- Tle 8 Developmental Changes: Six Major Stages in Human Life PubertyDocument4 pagesTle 8 Developmental Changes: Six Major Stages in Human Life PubertyOliver TabagNo ratings yet
- TroubleDocument15 pagesTroubleOliver TabagNo ratings yet
- O Shopping PDFDocument2 pagesO Shopping PDFOliver TabagNo ratings yet
- TotilacDocument4 pagesTotilacOliver Tabag100% (2)
- Spanish Finals 8-1591766528Document3 pagesSpanish Finals 8-1591766528Oliver TabagNo ratings yet
- Red Alert 2 Yuri's Revenge 1.001Document1 pageRed Alert 2 Yuri's Revenge 1.001Oliver TabagNo ratings yet
- Why Do We Study History?: Unit: Introduction To History Name: - Section: Class Notes DateDocument4 pagesWhy Do We Study History?: Unit: Introduction To History Name: - Section: Class Notes DateOliver TabagNo ratings yet
- SerialDocument1 pageSerialhkss80No ratings yet
- Let Me Explain, Pierre Teilhard de Chardin Edt Jean-Peirre Demoulin (1817)Document188 pagesLet Me Explain, Pierre Teilhard de Chardin Edt Jean-Peirre Demoulin (1817)WaterwindNo ratings yet
- Q3 - WRITE SIMPLE STORY - Grade 3Document5 pagesQ3 - WRITE SIMPLE STORY - Grade 3Elimi Rebucas100% (4)
- Exadata PricelistDocument14 pagesExadata Pricelistمحب الحقNo ratings yet
- Types of Essay StructuresDocument6 pagesTypes of Essay StructuressccrNo ratings yet
- What Is Interaction Design?Document51 pagesWhat Is Interaction Design?Vairavel ChenniyappanNo ratings yet
- CQI-9v3Forms and Process TablesBDocument48 pagesCQI-9v3Forms and Process TablesBjkguru75No ratings yet
- Chapter 1 Addressing PeopleDocument13 pagesChapter 1 Addressing PeopleArdana PutraNo ratings yet
- Design and Implementation Of: Vending Machine Using Verilog HDLDocument2 pagesDesign and Implementation Of: Vending Machine Using Verilog HDLPrakhar KumarNo ratings yet
- TAFL EXTERNAL Practical LISTDocument2 pagesTAFL EXTERNAL Practical LISTcompiler&automataNo ratings yet
- Piercing The Veil of RealityDocument15 pagesPiercing The Veil of RealitymysticdreamNo ratings yet
- User Manual For Gemcom WhittleDocument3 pagesUser Manual For Gemcom WhittleYohanesPambudi0% (1)
- VCSS Power School Parent Quick GuideDocument7 pagesVCSS Power School Parent Quick GuideShannonNo ratings yet
- PR 2Document37 pagesPR 2Nestor DagandanNo ratings yet
- Slac 1 - Training Design-RpmsDocument3 pagesSlac 1 - Training Design-RpmsPaciano Padao DexieNo ratings yet
- Drawing Dragster With SketchUp (Tutorial)Document2 pagesDrawing Dragster With SketchUp (Tutorial)kmardock064100% (1)
- Combine PDFDocument3 pagesCombine PDFkoushikNo ratings yet
- Progressive Printing: Book 4Document25 pagesProgressive Printing: Book 4Jacob PruittNo ratings yet
- IEEE STD IEEE-1346-1998 - IEEE Recommenden Practice For Ev. EPS. Compability PDFDocument45 pagesIEEE STD IEEE-1346-1998 - IEEE Recommenden Practice For Ev. EPS. Compability PDFCarlos Gabriel Quintero RodríguezNo ratings yet
- EDU104 Lec Notes 9Document4 pagesEDU104 Lec Notes 9Bagas GesangNo ratings yet
- Biodiversity Hotspot: MadagascarDocument1 pageBiodiversity Hotspot: MadagascarMr Cornish100% (2)
- IELTS4 Answer KeysDocument2 pagesIELTS4 Answer Keysmayura surangaNo ratings yet
- ConstructionDocument5 pagesConstructionapi-407289133No ratings yet
- 004Document119 pages004svsreeramaNo ratings yet
- QEI SCADA SystemsDocument1 pageQEI SCADA SystemsLee PearceNo ratings yet
- Ado.netDocument24 pagesAdo.netExample ExNo ratings yet
- The InterviewDocument2 pagesThe Interviewirfanoushad15No ratings yet