Professional Documents
Culture Documents
M.SAIDI 2023.09.13 09.58.13 Details
Uploaded by
Laboratorium RSI Sultan Agung Banjarbaru0 ratings0% found this document useful (0 votes)
8 views2 pagesOriginal Title
M.SAIDI_2023.09.13_09.58.13_details
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
8 views2 pagesM.SAIDI 2023.09.13 09.58.13 Details
Uploaded by
Laboratorium RSI Sultan Agung BanjarbaruCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
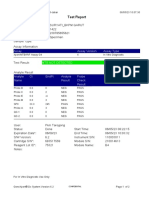
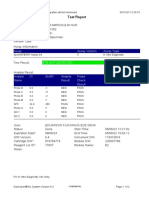
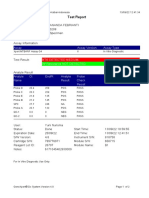
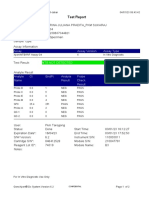
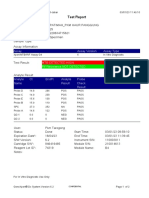
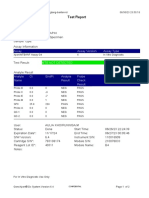
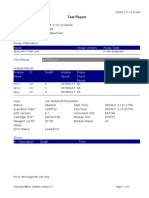
843558-Puskesmas LUT-Banjarbaru-Indonesia 13/09/23 12:04:19
Test Report
Patient ID: 6307081703030001
Patient ID 2: 1279
Sample ID: M.SAIDI
Test Type: Specimen
Sample Type:
Assay Information
Assay Assay Version Assay Type
Xpert MTB-RIF Ultra 4 In Vitro Diagnostic
Test Result: MTB NOT DETECTED
-
Analyte Result
Analyte Ct EndPt Analyte Probe
Name Result Check
Result
SPC 26.6 145 PASS PASS
IS1081- 0.0 9 FAIL PASS
IS6110
rpoB1 0.0 2 INVALID PASS
rpoB2 0.0 2 INVALID PASS
rpoB3 0.0 6 INVALID PASS
rpoB4 0.0 14 INVALID PASS
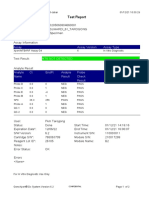
User: puskesmas LUT
Status: Done Start Time: 13/09/23 09:58:13
Expiration Date*: 27/10/24 End Time: 13/09/23 11:03:54
S/W Version: 5.1 Instrument S/N: 843558
Cartridge S/N*: 927442728 Module S/N: 725318
Reagent Lot ID*: 51701 Module Name: B4
Notes:
Error Status: OK
-
Errors
<None>
For In Vitro Diagnostic Use Only.
GeneXpert® Dx System Version 5.1 Page 1 of 2
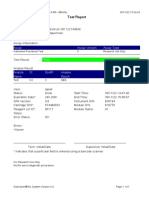
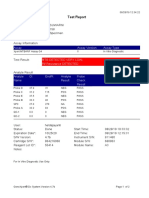
843558-Puskesmas LUT-Banjarbaru-Indonesia 13/09/23 12:04:19
Test Report
Tech. Initial/Date Supervisor Initial/Date
* indicates that a particular field is entered using a barcode scanner
For In Vitro Diagnostic Use Only.
GeneXpert® Dx System Version 5.1 Page 2 of 2
You might also like
- Interpretation: L55 - PSC Jayanagar Home Visit Municipal New No.42, Old No 825, Ground, W K P Road, 7Th Block, JayanagarDocument2 pagesInterpretation: L55 - PSC Jayanagar Home Visit Municipal New No.42, Old No 825, Ground, W K P Road, 7Th Block, JayanagardrmohangNo ratings yet
- Rsi Sa 12-10-2023Document6 pagesRsi Sa 12-10-2023Laboratorium RSI Sultan Agung BanjarbaruNo ratings yet
- Nurbaya 2023.02.02 14.49.06 DetailsDocument2 pagesNurbaya 2023.02.02 14.49.06 DetailsRahmatul LailiNo ratings yet
- 2023.05.06 08.22.15 DetailsDocument2 pages2023.05.06 08.22.15 Detailsakreditasi tarogong 2023No ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay Typeyossy aprillyaNo ratings yet
- 2023.01.03 08.06.58 DetailsDocument2 pages2023.01.03 08.06.58 Detailsakreditasi tarogong 2023No ratings yet
- Cihideung TCM DetailsDocument10 pagesCihideung TCM Detailsmulyadi diningrumNo ratings yet
- Tamron IDocument2 pagesTamron IEri OiNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay TypesetyawanankesNo ratings yet
- SUKARMINI-DPM DR ABDUL GHOFIRDocument2 pagesSUKARMINI-DPM DR ABDUL GHOFIRlaboratorium mitra utamaNo ratings yet
- Ananda FebriantiDocument2 pagesAnanda FebriantiInternis RsuyarsiNo ratings yet
- PKM BetoambariDocument6 pagesPKM Betoambarinur liylaNo ratings yet
- 2023.01.03 16.12.27 DetailsDocument2 pages2023.01.03 16.12.27 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 09.59.10 DetailsDocument2 pages2023.01.03 09.59.10 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 15.25.33 DetailsDocument2 pages2023.01.03 15.25.33 Detailsakreditasi tarogong 2023No ratings yet
- NANI K 46TH PASUNDAN 2021.12.01 08.36.02 DetailsDocument4 pagesNANI K 46TH PASUNDAN 2021.12.01 08.36.02 Detailsakreditasi tarogong 2023No ratings yet
- Hasim. S1Document2 pagesHasim. S1pkmsilo1No ratings yet
- 2023.01.03 11.48.53 DetailsDocument2 pages2023.01.03 11.48.53 Detailsakreditasi tarogong 2023No ratings yet
- IWA 29TH PKM TRG 2021.12.01 08.35.17 DetailsDocument4 pagesIWA 29TH PKM TRG 2021.12.01 08.35.17 Detailsakreditasi tarogong 2023No ratings yet
- APIHDocument2 pagesAPIHpuskesmas cigeulisNo ratings yet
- 3Document6 pages3Jose F. Ramirez MendozaNo ratings yet
- SUHARDI 61 TAROGONG 2021.12.01 14.18.16 DetailsDocument2 pagesSUHARDI 61 TAROGONG 2021.12.01 14.18.16 Detailsakreditasi tarogong 2023No ratings yet
- Bungga Hobrouw (R.anak)Document2 pagesBungga Hobrouw (R.anak)lukas mansnandifuNo ratings yet
- Instrum 301122144638 2022.11.30 14.47.40 DetailsDocument1 pageInstrum 301122144638 2022.11.30 14.47.40 DetailsLabovida RoraimaNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument6 pagesTest Report: Assay Assay Version Assay TypeJose F. Ramirez MendozaNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument3 pagesTest Report: Assay Assay Version Assay TypeAlex MoralesNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument7 pagesTest Report: Assay Assay Version Assay TyperosmayaniimutzNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay TypeMelania Dessy Savitri SunarjadiNo ratings yet
- Oft34buc2lq1c4zzfauf25qxDocument2 pagesOft34buc2lq1c4zzfauf25qxSumit Agarwal0% (1)
- S62 - Lpl-Nagpur - 2 Plot No. 7, Ground Floor Hotel Sunny Int Besides Gujrati Samaj Bhavan, Dhantoli Nagpur 440012Document2 pagesS62 - Lpl-Nagpur - 2 Plot No. 7, Ground Floor Hotel Sunny Int Besides Gujrati Samaj Bhavan, Dhantoli Nagpur 440012prachi jaiswalNo ratings yet
- Diptajyoti Mitra ReportsDocument2 pagesDiptajyoti Mitra ReportsBuddhadeb ChatterjeeNo ratings yet
- BAN63C25302981625435Document3 pagesBAN63C25302981625435sanjeevbiradar121No ratings yet
- BAN63C25302981625499Document3 pagesBAN63C25302981625499sanjeevbiradar121No ratings yet
- Pt. Pathlab Indonesia: (Laboratorium Klinik)Document1 pagePt. Pathlab Indonesia: (Laboratorium Klinik)Aghiest Utungga Al BirruNo ratings yet
- MayaDocument1 pageMayaRoshan RayNo ratings yet
- QT 0028767 PDFDocument12 pagesQT 0028767 PDFLisa FebriyantiNo ratings yet
- 0001ba008448 1 F1Document1 page0001ba008448 1 F1Ankush SainiNo ratings yet
- Molecular Biology: Results & Unit Reference Value Test DescriptionDocument1 pageMolecular Biology: Results & Unit Reference Value Test DescriptionLibu GeorgebabuNo ratings yet
- GPR Survey ProcedureDocument10 pagesGPR Survey Proceduresaffririzal4237No ratings yet
- Name Received Collected Dummy Z839: InterpretationDocument2 pagesName Received Collected Dummy Z839: Interpretationcra storeNo ratings yet
- Client: Pt. STBC Location: Autoclave 3 & 4 Report No.: 001-PAUT/RBT-STBC/XI/2022Document60 pagesClient: Pt. STBC Location: Autoclave 3 & 4 Report No.: 001-PAUT/RBT-STBC/XI/2022Rizal HidayatullahNo ratings yet
- TN039C56226475386137 RLSDocument3 pagesTN039C56226475386137 RLSnithya nithya0% (1)
- S53 - FPSC Batla House: Patientreportscsuperpanel - SP - General - Template01 - SC (Version: 7)Document2 pagesS53 - FPSC Batla House: Patientreportscsuperpanel - SP - General - Template01 - SC (Version: 7)anggita wahyu supraptiNo ratings yet
- Jytszb-R12-2100610 en 300328 WifiDocument77 pagesJytszb-R12-2100610 en 300328 WifionallpelinNo ratings yet
- AryaDocument2 pagesAryaRoob HoodNo ratings yet
- Bhyoopefc3z2xyurq0i5xfscDocument2 pagesBhyoopefc3z2xyurq0i5xfscNimit JainNo ratings yet
- Dipali Oraon - RT-PCR ReportDocument2 pagesDipali Oraon - RT-PCR ReportHimanshu TaterNo ratings yet
- Wang Binzhang - yDocument1 pageWang Binzhang - yDeffa Ajjah OfficialNo ratings yet
- CT100B System User ManualDocument22 pagesCT100B System User ManualJose RojasNo ratings yet
- D-37/1, TTC MIDC, Turbhe, Navi Mumbai-400 703: Processed At: ThyrocareDocument3 pagesD-37/1, TTC MIDC, Turbhe, Navi Mumbai-400 703: Processed At: ThyrocareNaitik N ShahNo ratings yet
- Plot No.428, Phase-IV, Udyog Vihar, Gurgaon, Haryana - 122 015Document3 pagesPlot No.428, Phase-IV, Udyog Vihar, Gurgaon, Haryana - 122 015Asit ANo ratings yet
- S81 - Krishna Diagnostics Harmu, by Pass Road, Near Sahjanand Chowk, Opp Durga Mandir Ranchi - 2Document2 pagesS81 - Krishna Diagnostics Harmu, by Pass Road, Near Sahjanand Chowk, Opp Durga Mandir Ranchi - 2AlokNo ratings yet
- Notification On University Website FinalDocument4 pagesNotification On University Website FinalRajendra Prasad R SNo ratings yet
- Alkhidmat Diagnostic Center Blood Bank: Virology & Genetic LabDocument1 pageAlkhidmat Diagnostic Center Blood Bank: Virology & Genetic LabZubair RajpootNo ratings yet
- Jinnah Central Lab: Jinnah Hospital & AIMC Lahore, PakistanDocument2 pagesJinnah Central Lab: Jinnah Hospital & AIMC Lahore, Pakistanafshan liaqatNo ratings yet
- Covid-19 Qualitative PCR Not Detected Target Gene CT Value: D-37/1, TTC MIDC, Turbhe, Navi Mumbai-400 703Document5 pagesCovid-19 Qualitative PCR Not Detected Target Gene CT Value: D-37/1, TTC MIDC, Turbhe, Navi Mumbai-400 703Kirti SuryawanshiNo ratings yet
- Comba ReportDocument51 pagesComba Reportculeros1No ratings yet
- Plot No.428, Phase-IV, Udyog Vihar, Gurgaon, Haryana - 122 015Document3 pagesPlot No.428, Phase-IV, Udyog Vihar, Gurgaon, Haryana - 122 015Sourav ChakrabotyNo ratings yet
- Sodium, Blood ,: Test Name Result Biological Ref. Interval MethodDocument3 pagesSodium, Blood ,: Test Name Result Biological Ref. Interval MethodPantha BiswasNo ratings yet