Professional Documents
Culture Documents
2023.01.03 09.59.10 Details
Uploaded by
akreditasi tarogong 20230 ratings0% found this document useful (0 votes)
12 views2 pagesOriginal Title
220634715621_2023.01.03_09.59.10_details
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
12 views2 pages2023.01.03 09.59.10 Details
Uploaded by
akreditasi tarogong 2023Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
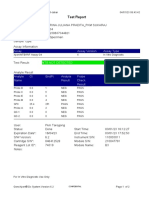
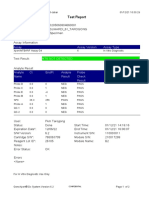
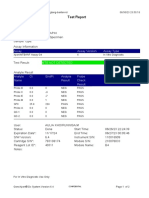
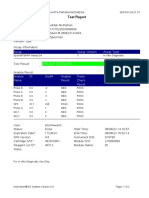
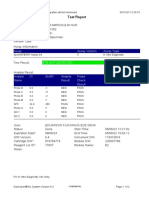
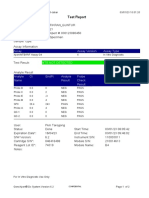
110003011-PKM Tarogong-Garut-Jabar 03/01/23 11:49:16
Test Report
Patient ID: PATIMAH_PKM HAUR PANGGUNG
Patient ID 2: 25
Sample ID: 220634715621
Test Type: Specimen
Sample Type:
Assay Information
Assay Assay Version Assay Type
Xpert MTB-RIF Assay G4 6 In Vitro Diagnostic
Test Result: MTB DETECTED HIGH;
Rif Resistance NOT DETECTED
-
Analyte Result
Analyte Ct EndPt Analyte Probe
Name Result Check
Result
Probe D 14.8 286 POS PASS
Probe C 14.9 238 POS PASS
Probe E 15.6 138 POS PASS
Probe B 15.9 119 POS PASS
SPC 27.3 252 NA PASS
Probe A 14.6 122 POS PASS
QC-1 0.0 0 NEG PASS
QC-2 0.0 0 NEG PASS
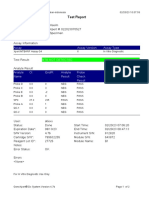
User: Pkm Tarogong
Status: Done Start Time: 03/01/23 09:59:10
Expiration Date*: 16/04/23 End Time: 03/01/23 11:39:45
S/W Version: 6.2 Instrument S/N: 110003011
Cartridge S/N*: 846416485 Module S/N: 210014613
Reagent Lot ID*: 74319 Module Name: B4
Notes:
For In Vitro Diagnostic Use Only.
GeneXpert® Dx System Version 6.2 CONFIDENTIAL Page 1 of 2
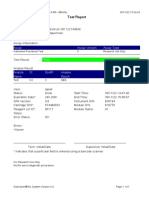
110003011-PKM Tarogong-Garut-Jabar 03/01/23 11:49:16
Test Report
Error Status: OK
-
Errors
<None>
Tech. Initial/Date Supervisor Initial/Date
* indicates that a particular field is entered using a barcode scanner
For In Vitro Diagnostic Use Only.
GeneXpert® Dx System Version 6.2 CONFIDENTIAL Page 2 of 2
You might also like
- 2023.01.03 08.06.58 DetailsDocument2 pages2023.01.03 08.06.58 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 11.48.53 DetailsDocument2 pages2023.01.03 11.48.53 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 16.12.27 DetailsDocument2 pages2023.01.03 16.12.27 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 15.25.33 DetailsDocument2 pages2023.01.03 15.25.33 Detailsakreditasi tarogong 2023No ratings yet
- NANI K 46TH PASUNDAN 2021.12.01 08.36.02 DetailsDocument4 pagesNANI K 46TH PASUNDAN 2021.12.01 08.36.02 Detailsakreditasi tarogong 2023No ratings yet
- 2023.05.06 08.22.15 DetailsDocument2 pages2023.05.06 08.22.15 Detailsakreditasi tarogong 2023No ratings yet
- SUHARDI 61 TAROGONG 2021.12.01 14.18.16 DetailsDocument2 pagesSUHARDI 61 TAROGONG 2021.12.01 14.18.16 Detailsakreditasi tarogong 2023No ratings yet
- IWA 29TH PKM TRG 2021.12.01 08.35.17 DetailsDocument4 pagesIWA 29TH PKM TRG 2021.12.01 08.35.17 Detailsakreditasi tarogong 2023No ratings yet
- Cihideung TCM DetailsDocument10 pagesCihideung TCM Detailsmulyadi diningrumNo ratings yet
- APIHDocument2 pagesAPIHpuskesmas cigeulisNo ratings yet
- SUKARMINI-DPM DR ABDUL GHOFIRDocument2 pagesSUKARMINI-DPM DR ABDUL GHOFIRlaboratorium mitra utamaNo ratings yet
- Nurbaya 2023.02.02 14.49.06 DetailsDocument2 pagesNurbaya 2023.02.02 14.49.06 DetailsRahmatul LailiNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay Typeyossy aprillyaNo ratings yet
- Tamron IDocument2 pagesTamron IEri OiNo ratings yet
- PKM BetoambariDocument6 pagesPKM Betoambarinur liylaNo ratings yet
- Bungga Hobrouw (R.anak)Document2 pagesBungga Hobrouw (R.anak)lukas mansnandifuNo ratings yet
- 3Document6 pages3Jose F. Ramirez MendozaNo ratings yet
- Ananda FebriantiDocument2 pagesAnanda FebriantiInternis RsuyarsiNo ratings yet
- M.SAIDI 2023.09.13 09.58.13 DetailsDocument2 pagesM.SAIDI 2023.09.13 09.58.13 DetailsLaboratorium RSI Sultan Agung BanjarbaruNo ratings yet
- Rsi Sa 12-10-2023Document6 pagesRsi Sa 12-10-2023Laboratorium RSI Sultan Agung BanjarbaruNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument6 pagesTest Report: Assay Assay Version Assay TypeJose F. Ramirez MendozaNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay TypeMelania Dessy Savitri SunarjadiNo ratings yet
- Hasim. S1Document2 pagesHasim. S1pkmsilo1No ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay TypesetyawanankesNo ratings yet
- Instrum 301122144638 2022.11.30 14.47.40 DetailsDocument1 pageInstrum 301122144638 2022.11.30 14.47.40 DetailsLabovida RoraimaNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument3 pagesTest Report: Assay Assay Version Assay TypeAlex MoralesNo ratings yet
- PCRDocument6 pagesPCRJose F. Ramirez MendozaNo ratings yet
- 31-03-2020 C5 HRSG - LP Reedwater To HRSG DRUM Level Control Valve - PTDocument3 pages31-03-2020 C5 HRSG - LP Reedwater To HRSG DRUM Level Control Valve - PTDave CheungNo ratings yet
- 0001ba008448 1 F1Document1 page0001ba008448 1 F1Ankush SainiNo ratings yet
- Meghna PVC LTD (Pet Division) QAC Department Shift Chemist Log BookDocument5 pagesMeghna PVC LTD (Pet Division) QAC Department Shift Chemist Log Bookswapon kumar shillNo ratings yet
- Qa Test Format X RayDocument9 pagesQa Test Format X RayNIKHIL VERMANo ratings yet
- Akanksha Covid19 JanDocument3 pagesAkanksha Covid19 JanAkanksha MehtaNo ratings yet
- A-Star Testing & Inspection (S) Pte LTD: Magnetic Particle Testing ReportDocument4 pagesA-Star Testing & Inspection (S) Pte LTD: Magnetic Particle Testing ReportHari KarthickNo ratings yet
- Gotaq Probe 1-Step RT-QPCR System: Technical ManualDocument11 pagesGotaq Probe 1-Step RT-QPCR System: Technical ManualJaimeAdolfoAndresBlasNo ratings yet
- Comba ReportDocument51 pagesComba Reportculeros1No ratings yet
- Wavelength accuracy test passesDocument2 pagesWavelength accuracy test passescarlos germanNo ratings yet
- NM002C23421596794477 - RLS 3 Converted 2Document3 pagesNM002C23421596794477 - RLS 3 Converted 2pravins93No ratings yet
- COVID-19 PCR Test Report for Saneer Puthiya ValappilDocument1 pageCOVID-19 PCR Test Report for Saneer Puthiya ValappilSANEER P VNo ratings yet
- WO 2626 - PO 4504180804 - General Inspection - PGCDocument75 pagesWO 2626 - PO 4504180804 - General Inspection - PGCMatheus Fabrício TeixeiraNo ratings yet
- COVID-19 Test Report for Ms. MAYA DEVKOTADocument1 pageCOVID-19 Test Report for Ms. MAYA DEVKOTARoshan RayNo ratings yet
- REPORT 2016 06 23 1646 g14Document3 pagesREPORT 2016 06 23 1646 g14Asela BamunuarachchiNo ratings yet
- Sars-Cov2 (Covid-19) Real Time RT PCR TestDocument2 pagesSars-Cov2 (Covid-19) Real Time RT PCR TestGEO MERINNo ratings yet
- CNT230022-5 C3 HRSG LP Drum - MTreportDocument6 pagesCNT230022-5 C3 HRSG LP Drum - MTreportDave CheungNo ratings yet
- Certificado Test ReportDocument28 pagesCertificado Test ReportdronebessaNo ratings yet
- Covid-19 Qualitative PCR Target Gene CT Value Detected 30.44Document4 pagesCovid-19 Qualitative PCR Target Gene CT Value Detected 30.44Kiran ShelarNo ratings yet
- Mpi D212Document1 pageMpi D212K.s. Raghavendra KumarNo ratings yet
- Glifosato Nppe-19573Document2 pagesGlifosato Nppe-19573AS AdivNo ratings yet
- OVP - OQ Test Protocol: Overall Test Result PASSEDDocument8 pagesOVP - OQ Test Protocol: Overall Test Result PASSEDAli RizviNo ratings yet
- Worksheet - PTDocument5 pagesWorksheet - PTDave CheungNo ratings yet
- SARS-CoV-2 (Covid-19) RT-PCR test report showing negative resultDocument1 pageSARS-CoV-2 (Covid-19) RT-PCR test report showing negative resultPriyansh PatelNo ratings yet
- Car Charger CE-EMC ReportDocument21 pagesCar Charger CE-EMC Reportgqg9sw6kbgNo ratings yet
- QT 0028767 PDFDocument12 pagesQT 0028767 PDFLisa FebriyantiNo ratings yet
- VARGA ResultsDocument3 pagesVARGA ResultsCocos MirelNo ratings yet
- RTTE报备报告E903 en 62479 FOR 300328Document12 pagesRTTE报备报告E903 en 62479 FOR 300328Signzworld UkcutterNo ratings yet
- Pt. Pathlab Indonesia: (Laboratorium Klinik)Document1 pagePt. Pathlab Indonesia: (Laboratorium Klinik)Aghiest Utungga Al BirruNo ratings yet
- Alkhidmat Diagnostic Center Blood Bank: Virology & Genetic LabDocument1 pageAlkhidmat Diagnostic Center Blood Bank: Virology & Genetic LabZubair RajpootNo ratings yet
- MIghty Machine RejectDocument1 pageMIghty Machine RejectMohammad Shanawaz MNo ratings yet
- COVID-19 PCR test detectedDocument3 pagesCOVID-19 PCR test detectedKAMAL KANTNo ratings yet
- Transistor Electronics: Use of Semiconductor Components in Switching OperationsFrom EverandTransistor Electronics: Use of Semiconductor Components in Switching OperationsRating: 1 out of 5 stars1/5 (1)
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- Xpert M 030123080450 2023.01.03 08.05.42 DetailsDocument2 pagesXpert M 030123080450 2023.01.03 08.05.42 Detailsakreditasi tarogong 2023No ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet
- AHMAD F N 24 TAROGONG 2021.12.01 14.18.53 DetailsDocument2 pagesAHMAD F N 24 TAROGONG 2021.12.01 14.18.53 Detailslaboratorium puskesmas tarogongNo ratings yet